Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors
- PMID: 10077673
- PMCID: PMC15931
- DOI: 10.1073/pnas.96.6.3269
Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors
Abstract
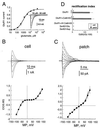
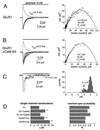
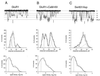
The ability of central glutamatergic synapses to change their strength in response to the intensity of synaptic input, which occurs, for example, in long-term potentiation (LTP), is thought to provide a cellular basis for memory formation and learning. LTP in the CA1 field of the hippocampus requires activation of Ca2+/calmodulin-kinase II (CaM-KII), which phosphorylates Ser-831 in the GluR1 subunit of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate glutamate receptor (AMPA-R), and this activation/phosphorylation is thought to be a postsynaptic mechanism in LTP. In this study, we have identified a molecular mechanism by which CaM-KII potentiates AMPA-Rs. Coexpression in HEK-293 cells of activated CaM-KII with GluR1 did not affect the glutamate affinity of the receptor, the kinetics of desensitization and recovery, channel rectification, open probability, or gating. Single-channel recordings identified multiple conductance states for GluR1, and coexpression with CaM-KII or a mutation of Ser-831 to Asp increased the contribution of the higher conductance states. These results indicate that CaM-KII can mediate plasticity at glutamatergic synapses by increasing single-channel conductance of existing functional AMPA-Rs or by recruiting new high-conductance-state AMPA-Rs.
Figures

References
-
- Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. - PubMed
-
- Nicoll R A, Malenka R. Nature (London) 1995;377:115–118. - PubMed
-
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. J Biol Chem. 1993;268:7863–7867. - PubMed
-
- Fukunaga K, Muller D, Miyamoto E. J Biol Chem. 1995;270:6119–6124. - PubMed
-
- Giese K P, Fedorov N B, Filipkowski R K, Silva A J. Science. 1998;279:870–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

