Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production
- PMID: 10079098
- PMCID: PMC408149
- DOI: 10.1172/JCI5909
Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production
Abstract
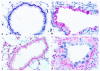
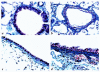
Interleukin (IL)-13 is a pleiotropic cytokine produced in large quantities by activated CD4(+) Th2 lymphocytes. To define further its potential in vivo effector functions, the Clara cell 10-kDa protein promoter was used to express IL-13 selectively in the lung, and the phenotype of the resulting transgenic mice was characterized. In contrast to transgene-negative littermates, the lungs of transgene-positive mice contained an inflammatory response around small and large airways and in the surrounding parenchyma. It was mononuclear in nature and contained significant numbers of eosinophils and enlarged and occasionally multinucleated macrophages. Airway epithelial cell hypertrophy, mucus cell metaplasia, the hyperproduction of neutral and acidic mucus, the deposition of Charcot-Leyden-like crystals, and subepithelial airway fibrosis were also prominently noted. Eotaxin protein and mRNA were also present in large quantities in the lungs of the transgene-positive, but not the transgene-negative, mice. IL-4, IL-5, granulocyte-macrophage colony-stimulating factor, and monocyte chemoattractant protein-5 were not similarly detected. Physiological evaluations revealed significant increases in baseline airways resistance and airways hyperresponsiveness (AHR) to methacholine in transgene-positive animals. Thus, the targeted pulmonary expression of IL-13 causes a mononuclear and eosinophilic inflammatory response, mucus cell metaplasia, the deposition of Charcot-Leyden-like crystals, airway fibrosis, eotaxin production, airways obstruction, and nonspecific AHR. IL-13 may play an important role in the pathogenesis of similar responses in asthma or other Th2-polarized tissue responses.
Figures
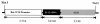
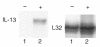



References
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. - PubMed
-
- Moller DR, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–4960. - PubMed
-
- Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4R alpha–deficient and IL-4–deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

