Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin
- PMID: 10079109
- PMCID: PMC408153
- DOI: 10.1172/JCI6042
Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin
Abstract
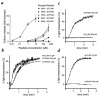
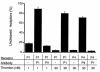
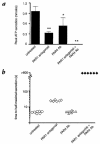
Because of the role of thrombin and platelets in myocardial infarction and other pathological processes, identifying and blocking the receptors by which thrombin activates platelets has been an important goal. Three protease-activated receptors (PARs) for thrombin -- PAR1, PAR3, and PAR4 -- are now known. PAR1 functions in human platelets, and the recent observation that a PAR4-activating peptide activates human platelets suggests that PAR4 also acts in these cells. Whether PAR1 and PAR4 account for activation of human platelets by thrombin, or whether PAR3 or still other receptors contribute, is unknown. We have examined the roles of PAR1, PAR3, and PAR4 in platelets. PAR1 and PAR4 mRNA and protein were detected in human platelets. Activation of either receptor was sufficient to trigger platelet secretion and aggregation. Inhibition of PAR1 alone by antagonist, blocking antibody, or desensitization blocked platelet activation by 1 nM thrombin but only modestly attenuated platelet activation by 30 nM thrombin. Inhibition of PAR4 alone using a blocking antibody had little effect at either thrombin concentration. Strikingly, simultaneous inhibition of both PAR1 and PAR4 virtually ablated platelet secretion and aggregation, even at 30 nM thrombin. These observations suggest that PAR1 and PAR4 account for most, if not all, thrombin signaling in platelets and that antagonists that block these receptors might be useful antithrombotic agents.
Figures




References
-
- Davey M, Luscher E. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967;216:857–858. - PubMed
-
- Berndt, M., and Phillips, D. 1981. Platelet membrane proteins: composition and receptor function. In Platelets in biology and pathology. J.L. Gordon, editor. Elsevier. Amsterdam, the Netherlands. 43–74.
-
- Vu T-KH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. - PubMed
-
- Rasmussen UB, et al. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. - PubMed
-
- Vu T-KH, Wheaton VI, Hung DT, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

