Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase
- PMID: 10079111
- PMCID: PMC408139
- DOI: 10.1172/JCI4829
Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase
Abstract
Hypercholesterolemia is a central pathogenic factor of endothelial dysfunction caused in part by an impairment of endothelial nitric oxide (NO) production through mechanisms that remain poorly characterized. The activity of the endothelial isoform of NO synthase (eNOS) was recently shown to be modulated by its reciprocal interactions with the stimulatory Ca2+-calmodulin complex and the inhibitory protein caveolin. We examined whether hypercholesterolemia may reduce NO production through alteration of this regulatory equilibrium. Bovine aortic endothelial cells were cultured in the presence of serum obtained from normocholesterolemic (NC) or hypercholesterolemic (HC) human volunteers. Exposure of endothelial cells to the HC serum upregulated caveolin abundance without any measurable effect on eNOS protein levels. This effect of HC serum was associated with an impairment of basal NO release paralleled by an increase in inhibitory caveolin-eNOS complex formation. Similar treatment with HC serum significantly attenuated the NO production stimulated by the calcium ionophore A23187. Accordingly, higher calmodulin levels were required to disrupt the enhanced caveolin-eNOS heterocomplex from HC serum-treated cells. Finally, cell exposure to the low-density lipoprotein (LDL) fraction alone dose-dependently reproduced the inhibition of basal and stimulated NO release, as well as the upregulation of caveolin expression and its heterocomplex formation with eNOS, which were unaffected by cotreatment with antioxidants. Together, our data establish a new mechanism for the cholesterol-induced impairment of NO production through the modulation of caveolin abundance in endothelial cells, a mechanism that may participate in the pathogenesis of endothelial dysfunction and the proatherogenic effects of hypercholesterolemia.
Figures
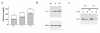


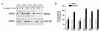

Similar articles
-
Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions.Biochemistry. 1998 Jan 6;37(1):193-200. doi: 10.1021/bi972307p. Biochemistry. 1998. PMID: 9425039
-
Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance.Circulation. 2001 Jan 2;103(1):113-8. doi: 10.1161/01.cir.103.1.113. Circulation. 2001. PMID: 11136695
-
The calcium channel blocker amlodipine promotes the unclamping of eNOS from caveolin in endothelial cells.Cardiovasc Res. 2006 Aug 1;71(3):478-85. doi: 10.1016/j.cardiores.2006.04.013. Epub 2006 Apr 27. Cardiovasc Res. 2006. PMID: 16814758
-
Cholesterol-dependent regulation of nitric oxide production: potential role in atherosclerosis.Nutr Rev. 1999 Sep;57(9 Pt 1):279-82. doi: 10.1111/j.1753-4887.1999.tb01812.x. Nutr Rev. 1999. PMID: 10568338 Review.
-
Signal transduction of eNOS activation.Cardiovasc Res. 1999 Aug 15;43(3):532-41. doi: 10.1016/s0008-6363(99)00094-2. Cardiovasc Res. 1999. PMID: 10690325 Review.
Cited by
-
Development of a novel Guinea Pig model producing transgenerational endothelial transcriptional changes driven by maternal food restriction and a second metabolic insult of high fat diet.Front Physiol. 2023 Oct 24;14:1266444. doi: 10.3389/fphys.2023.1266444. eCollection 2023. Front Physiol. 2023. PMID: 37942229 Free PMC article.
-
Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway.Sci Rep. 2020 Oct 13;10(1):17078. doi: 10.1038/s41598-020-74022-y. Sci Rep. 2020. PMID: 33051481 Free PMC article.
-
Caveolin-2-deficient mice show increased sensitivity to endotoxemia.Cell Cycle. 2011 Jul 1;10(13):2151-61. doi: 10.4161/cc.10.13.16234. Epub 2011 Jul 1. Cell Cycle. 2011. PMID: 21670588 Free PMC article.
-
Maternal supraphysiological hypercholesterolemia associates with endothelial dysfunction of the placental microvasculature.Sci Rep. 2018 May 16;8(1):7690. doi: 10.1038/s41598-018-25985-6. Sci Rep. 2018. PMID: 29769708 Free PMC article.
-
Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice.Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11375-80. doi: 10.1073/pnas.172360799. Epub 2002 Aug 12. Proc Natl Acad Sci U S A. 2002. PMID: 12177436 Free PMC article.
References
-
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. - PubMed
-
- Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–1867. - PubMed
-
- Seiler C, Hess OM, Buechi M, Suter TM, Krayenbuehl HP. Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary arteries. Circulation. 1993;88:2139–2148. - PubMed
-
- Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

