Murine cytomegalovirus immediate-early promoter directs astrocyte-specific expression in transgenic mice
- PMID: 10079251
- PMCID: PMC1866421
- DOI: 10.1016/S0002-9440(10)65320-5
Murine cytomegalovirus immediate-early promoter directs astrocyte-specific expression in transgenic mice
Abstract
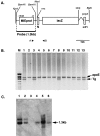
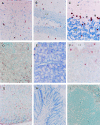
Murine cytomegalovirus (MCMV), which causes acute, latent, and persistent infection of the natural host, is used as an animal model of human cytomegalovirus (HCMV) infection. Transcription of MCMV immediate-early (IE) genes is required for expression of the early and late genes and is dependent on host cell transcription factors. Cell-type-specific expression activity of the MCMV IE promoter was analyzed in transgenic mice generated with the major IE (MIE) enhancer/promoter involving nucleotides -1343 to -6 (1338 bp) connected to the reporter gene lacZ. Distinct expression was observed in the brain, kidneys, stomach, and skeletal muscles. Weak expression was observed in a portion of the parenchymal cells of the salivary glands and pancreas, and expression was hardly detected in the lungs, intestine, or immune and hematopoietic organs such as the thymus, spleen, lymph nodes, and bone marrow. The spectrum of organs positive for expression was narrower than that of the HCMV MIE promoter-lacZ transgenic mice reported previously and showed a greater degree of cell-type specificity. Interestingly, astrocyte-specific expression of the transgene was observed in the brain and primary glial cultures from the transgenic mice by combination of beta-galactosidase (beta-Gal) expression and immunostaining for cell markers. However, the transgene was not expressed in neurons, oligodendroglia, microglia, or endothelial cells. Furthermore, the beta-Gal expression in glial cultures was stimulated significantly by MCMV infection or by addition of calcium ionophore. These observations indicated that expression activity of the MCMV IE promoter is strictly cell-type specific, especially astrocyte-specific in the brain. This specific pattern of activity is similar to that of natural HCMV infection in humans.
Figures


Similar articles
-
Activation of murine cytomegalovirus immediate-early promoter in cerebral ventricular zone and glial progenitor cells in transgenic mice.Glia. 2001 Jul;35(1):41-52. doi: 10.1002/glia.1069. Glia. 2001. PMID: 11424191
-
Activation of murine cytomegalovirus immediate-early promoter in mouse brain after transplantation of the neural stem cells.Acta Neuropathol. 2004 May;107(5):406-12. doi: 10.1007/s00401-004-0828-0. Epub 2004 Mar 23. Acta Neuropathol. 2004. PMID: 15042384
-
Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors.J Gen Virol. 1997 Jul;78 ( Pt 7):1653-61. doi: 10.1099/0022-1317-78-7-1653. J Gen Virol. 1997. PMID: 9225042
-
The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice.J Virol. 1996 May;70(5):3207-14. doi: 10.1128/JVI.70.5.3207-3214.1996. J Virol. 1996. PMID: 8627801 Free PMC article.
-
A model for reactivation of CMV from latency.J Clin Virol. 2002 Aug;25 Suppl 2:S123-36. doi: 10.1016/s1386-6532(02)00088-4. J Clin Virol. 2002. PMID: 12361763 Review.
Cited by
-
Tumorigenic potential of pituitary tumor transforming gene (PTTG) in vivo investigated using a transgenic mouse model, and effects of cross breeding with p53 (+/-) transgenic mice.BMC Cancer. 2012 Nov 20;12:532. doi: 10.1186/1471-2407-12-532. BMC Cancer. 2012. PMID: 23164239 Free PMC article.
-
Neurons differentially activate the herpes simplex virus type 1 immediate-early gene ICP0 and ICP27 promoters in transgenic mice.J Virol. 2002 Mar;76(5):2449-59. doi: 10.1128/jvi.76.5.2449-2459.2002. J Virol. 2002. PMID: 11836423 Free PMC article.
-
The transgenic ICP4 promoter is activated in Schwann cells in trigeminal ganglia of mice latently infected with herpes simplex virus type 1.J Virol. 2001 Nov;75(21):10401-8. doi: 10.1128/JVI.75.21.10401-10408.2001. J Virol. 2001. PMID: 11581408 Free PMC article.
-
Neuron-specific activation of murine cytomegalovirus early gene e1 promoter in transgenic mice.Am J Pathol. 2003 Aug;163(2):643-52. doi: 10.1016/S0002-9440(10)63691-7. Am J Pathol. 2003. PMID: 12875983 Free PMC article.
-
Maintenance of large numbers of virus genomes in human cytomegalovirus-infected T98G glioblastoma cells.J Virol. 2014 Apr;88(7):3861-73. doi: 10.1128/JVI.01166-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453365 Free PMC article.
References
-
- Weller TH: The cytomegalovirus: ubiquitous agents with protean clinical manifestation. N Engl J Med 1971, 285:203-214 - PubMed
-
- Becroft DMO: Prenatal cytomegalovirus infection: epidemiology, pathology, pathogenesis. Perspectives in Pediatric Pathology, Vol. 6: Infectious Diseases. Edited by HS Rosenberg and J Bernstein. New York, Masson Publishing USA, 1981, pp 203–241 - PubMed
-
- Bale JF: Human cytomegalovirus infection and disorders of the nervous system. Arch Neurol 1984, 41:310-320 - PubMed
-
- Ho M: Congenital and perinatal human cytomegalovirus infections. ed 2 Cytomegalovirus: Biology and Infection, 1991, :pp 205-227 Plenum Press, New York
-
- Britt WJ, Alford CA: Cytomegalovirus. Virology, ed 3. Edited by Fiels BN, Knipe DM, Howley PM. Philadelphia, Lippincott-Raven, 1996 pp 2493–2523
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

