Ki-A10, a germ cell nuclear antigen retained in a subset of germ cell-derived tumors
- PMID: 10079257
- PMCID: PMC1866408
- DOI: 10.1016/S0002-9440(10)65326-6
Ki-A10, a germ cell nuclear antigen retained in a subset of germ cell-derived tumors
Abstract
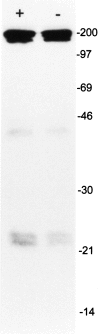
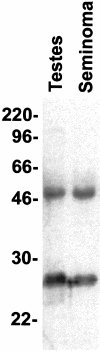
Monoclonal antibody Ki-A10 recognizes a nuclear antigen of 25 and 22 kd apparent molecular mass, which is abundantly expressed by immature gonocytes, spermatogonia, and spermatocytes, whereas it is absent in spermatids, spermatozoa, oocytes, and normal somatic tissues. In a broad spectrum of human cancers the antibody showed no reactivity except for a small subset of malignant lymphomas. Because of this restricted expression pattern, we examined 173 germ cell tumors and 18 sex cord stromal tumors immunohistochemically to assess the distribution of the Ki-A10 antigen. A strongly positive reaction was found in classic seminomas, dysgerminomas, spermatocytic seminomas, and the germ cell component of gonadoblastomas. Yolk sac tumors presented a heterogeneous reactivity pattern ranging from overall positivity to complete lack of antigen expression, and in three of eight choriocarcinomas, a few clusters of cytotrophoblast cells were strongly labeled. All other tumors, including Leydig and Sertoli cell tumors as well as placental tissue, were negative. Our findings suggest that specific germ cell antigens can be retained in germ cell tumors along particular differentiation pathways. Ki-A10 is the first marker that consistently labels spermatocytic seminoma, further confirming its germ cell origin and suggesting a close relationship to classic seminoma. The antibody may serve for diagnostic purposes and promises new insights into the process of germ cell differentiation and the development of germ cell-derived neoplasia.
Figures

Similar articles
-
Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues.Mod Pathol. 2005 Feb;18 Suppl 2:S61-79. doi: 10.1038/modpathol.3800310. Mod Pathol. 2005. PMID: 15761467 Review.
-
MAGE-1 and MAGE-3 tumor rejection antigens in human germ cell tumors.Mod Pathol. 1999 Oct;12(10):974-8. Mod Pathol. 1999. PMID: 10530563
-
Seminomas of the canine testis. Counterpart of spermatocytic seminoma of men?Lab Invest. 1994 Oct;71(4):490-6. Lab Invest. 1994. PMID: 7967505
-
Establishment of three monoclonal antibodies specific for prespermatogonia and intratubular malignant germ cells in humans.Lab Invest. 1997 Mar;76(3):427-38. Lab Invest. 1997. PMID: 9121125
-
[WHO classification of testicular tumors].Verh Dtsch Ges Pathol. 2002;86:67-75. Verh Dtsch Ges Pathol. 2002. PMID: 12647353 Review. German.
Cited by
-
Down-regulation of the cancer/testis antigen 45 (CT45) is associated with altered tumor cell morphology, adhesion and migration.Cell Commun Signal. 2013 Jun 10;11(1):41. doi: 10.1186/1478-811X-11-41. Cell Commun Signal. 2013. PMID: 23758873 Free PMC article.
-
Expression of cancer testis antigen CT45 in classical Hodgkin lymphoma and other B-cell lymphomas.Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3093-8. doi: 10.1073/pnas.0915050107. Epub 2010 Jan 26. Proc Natl Acad Sci U S A. 2010. PMID: 20133697 Free PMC article.
-
Multi-level Proteomics Identifies CT45 as a Chemosensitivity Mediator and Immunotherapy Target in Ovarian Cancer.Cell. 2018 Sep 20;175(1):159-170.e16. doi: 10.1016/j.cell.2018.08.065. Cell. 2018. PMID: 30241606 Free PMC article.
-
DNA hypomethylation-mediated activation of Cancer/Testis Antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer.Epigenetics. 2015;10(8):736-48. doi: 10.1080/15592294.2015.1062206. Epigenetics. 2015. PMID: 26098711 Free PMC article.
-
Chromosome X-encoded Cancer/Testis antigens are less frequently expressed in non-seminomatous germ cell tumors than in seminomas.Cancer Immun. 2013 May 10;13:10. Print 2013. Cancer Immun. 2013. PMID: 23885216 Free PMC article.
References
-
- Diehl V, Kirchner HH, Schaadt M, Fonatsch C, Stein H, Gerdes J, Boie C: Hodgkin’s disease: establishment and characterization of four in vitro cell lines. J Cancer Res Clin Oncol 1981, 101:111-124 - PubMed
-
- Skakkebaek NE: Possible carcinoma-in-situ of the testis. Lancet 1972, 2:516-517 - PubMed
-
- Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J: Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 1987, 10:19-28 - PubMed
-
- Oosterhuis JW, Castedo SM, de Jong B, Cornelisse CJ, Dam A, Sleijfer DT, Schraffordt Koops H: Ploidy of primary germ cell tumors of the testis: pathogenetic and clinical relevance. Lab Invest 1989, 60:14-21 - PubMed
-
- Damjanov I: Pathogenesis of testicular germ cell tumours. Eur Urol 1993, 23:2-5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

