Podocalyxin in rat platelets and megakaryocytes
- PMID: 10079259
- PMCID: PMC1866405
- DOI: 10.1016/S0002-9440(10)65328-X
Podocalyxin in rat platelets and megakaryocytes
Abstract
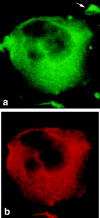
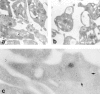
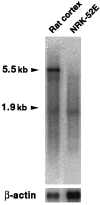
Podocalyxin is a membrane protein of rat podocytes and endothelial cells. It has not been described in other cell types, and no amino acid or DNA sequence data are available about it. Here we show that podocalyxin antigens are present in rat platelets and megakaryocytes. In resting platelets, the antigens are mainly intracellular but become surface exposed after thrombin stimulation, as shown by immunofluorescence and flow cytometry. By Western blotting, platelet podocalyxin has an apparent Mr of 140,000. Cytocentrifuge slides of rat bone marrow show that anti-podocalyxin antibodies recognize large polyploid cells also expressing CD62P, indicating that the cells are megakaryocytes. From a rat glomerular cDNA library we isolated a clone covering the carboxyl-terminal nucleotides of rat podocalyxin. Its putative transmembrane or intracellular domains are 100% or >93% identical, respectively, with the human and rabbit podocalyxin-like proteins. The truncated extracellular domain extends to include two of the four conserved cysteines shared by podocalyxin-like proteins. By Northern blotting, a 5.5-kb renal cortical transcript is seen. By in situ hybridization, cRNA probes recognize podocytes, endothelial cells, and megakaryocytes, and by reverse transcription polymerase chain reaction, platelets are shown to contain podocalyxin mRNA. Our results show that rat podocalyxin is a homologue of the previously cloned podocalyxin-like proteins and suggest that also in mammals podocalyxin has a role in hematopoiesis, as previously shown in the chicken.
Figures






Similar articles
-
Enhanced podocalyxin expression alters the structure of podocyte basal surface.J Cell Sci. 2004 Jul 1;117(Pt 15):3281-94. doi: 10.1242/jcs.01163. J Cell Sci. 2004. PMID: 15226400
-
Overexpression of podocalyxin in megakaryocytes and platelets decreases the bleeding time and enhances the agonist-induced aggregation of platelets.Thromb Res. 2010 Jun;125(6):e300-5. doi: 10.1016/j.thromres.2010.02.008. Epub 2010 Mar 12. Thromb Res. 2010. PMID: 20223501
-
Platelets contribute to circulating levels of bone sialoprotein in human.J Bone Miner Res. 1992 Jan;7(1):47-54. doi: 10.1002/jbmr.5650070108. J Bone Miner Res. 1992. PMID: 1549958
-
Role of platelets and megakaryocytes in adaptive immunity.Platelets. 2021 Apr 3;32(3):340-351. doi: 10.1080/09537104.2020.1786043. Epub 2020 Jun 27. Platelets. 2021. PMID: 32597341 Review.
-
The role of inflammation in regulating platelet production and function: Toll-like receptors in platelets and megakaryocytes.Thromb Res. 2010 Mar;125(3):205-9. doi: 10.1016/j.thromres.2009.11.004. Epub 2009 Nov 27. Thromb Res. 2010. PMID: 19945154 Free PMC article. Review.
Cited by
-
Cloning and expression of the rat nephrin homolog.Am J Pathol. 1999 Sep;155(3):907-13. doi: 10.1016/S0002-9440(10)65190-5. Am J Pathol. 1999. PMID: 10487848 Free PMC article.
-
Pleomorphic extra-renal manifestation of the glomerular podocyte marker podocalyxin in tissues of normal beagle dogs.Histochem Cell Biol. 2007 Apr;127(4):399-414. doi: 10.1007/s00418-006-0252-8. Epub 2006 Dec 19. Histochem Cell Biol. 2007. PMID: 17180683
-
Podocalyxin Expressed in Antigen Presenting Cells Promotes Interaction With T Cells and Alters Centrosome Translocation to the Contact Site.Front Immunol. 2022 May 31;13:835527. doi: 10.3389/fimmu.2022.835527. eCollection 2022. Front Immunol. 2022. PMID: 35711462 Free PMC article.
-
Identification and expression profiling of blood-brain barrier membrane proteins.J Neurochem. 2010 Feb;112(3):625-35. doi: 10.1111/j.1471-4159.2009.06481.x. Epub 2009 Nov 6. J Neurochem. 2010. PMID: 19895664 Free PMC article.
-
Emerging Role of Podocalyxin in the Progression of Mature B-Cell Non-Hodgkin Lymphoma.Cancers (Basel). 2020 Feb 8;12(2):396. doi: 10.3390/cancers12020396. Cancers (Basel). 2020. PMID: 32046309 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

