The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts
- PMID: 10079265
- PMCID: PMC1866402
- DOI: 10.1016/s0002-9440(10)65334-5
The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts
Abstract
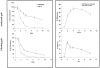
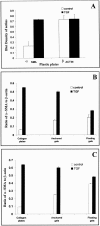
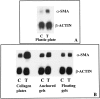
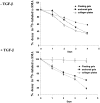
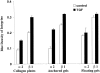
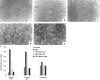
Wound contraction is mediated by myofibroblasts, specialized fibroblasts that appear in large numbers as the wound matures and when resistance to contractile forces increases. We considered that the regulation of myofibroblast differentiation by wound-healing cytokines may be dependent on the resistance of the connective tissue matrix to deformation. We examined transforming growth factor-beta1 (TGF-beta1) induction of the putative fibroblast contractile marker, alpha-smooth muscle actin (alpha-SMA), and the regulation of this process by the compliance of collagen substrates. Cells were cultured in three different types of collagen gels with wide variations of mechanical compliance as assessed by deformation testing. The resistance to collagen gel deformation determined the levels of intracellular tension as shown by staining for actin stress fibers. For cells plated on thin films of collagen-coated plastic (ie, minimal compliance and maximal intracellular tension), TGF-beta1 (10 ng/ml; 6 days) increased alpha-SMA protein content by ninefold as detected by Western blots but did not affect beta-actin content. Western blots of cells in anchored collagen gels (moderate compliance and tension) also showed a TGF-beta1-induced increase of alpha-SMA content, but the effect was greatly reduced compared with collagen-coated plastic (<3-fold increase). In floating collagen gels (high compliance and low tension), there were only minimal differences of alpha-SMA protein. Northern analyses for alpha-SMA and beta-actin indicated that TGF-beta1 selectively increased mRNA for alpha-SMA similar to the reported protein levels. In pulse-chase experiments, [35S]methionine-labeled intracellular alpha-SMA decayed most rapidly in floating gels, less rapidly in anchored gels, and not at all in collagen plates after TGF-beta1 treatment. TGF-beta1 increased alpha2 and beta1 integrin content by 50% in cells on collagen plates, but the increase was less marked on anchored gels and was undetectable in floating gels. When intracellular tension on collagen substrates was reduced by preincubating cells with blocking antibodies to the alpha2 and beta1 integrin subunits, TGF-beta1 failed to increase alpha-SMA protein content in all three types of collagen matrices. These data indicate that TGF-beta1-induced increases of alpha-SMA content are dependent on the resistance of the substrate to deformation and that the generation of intracellular tension is a central determinant of contractile cytoskeletal gene expression.
Figures


References
-
- Skalli O, Gabbiani G: The biology of the myofibroblast. Molecular and Cellular Biology of Wound Repair. Edited by Clark RAF, Henson PM. New York, Plenum, 1996
-
- Darby I, Skalli O, Gabbiani G: α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990, 63:21-29 - PubMed
-
- Burridge K: Are stress fibers contractile? Nature 1981, 249:61-62 - PubMed
-
- Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

