Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains
- PMID: 10082512
- PMCID: PMC84039
- DOI: 10.1128/MCB.19.4.2465
Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains
Abstract
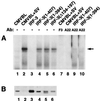
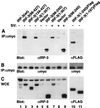
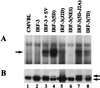
The interferon regulatory factor 3 (IRF-3) gene encodes a 55-kDa protein which is expressed constitutively in all tissues. In unstimulated cells, IRF-3 is present in an inactive cytoplasmic form; following Sendai virus infection, IRF-3 is posttranslationally modified by protein phosphorylation at multiple serine and threonine residues located in the carboxy terminus. Virus-induced phosphorylation of IRF-3 leads to cytoplasmic to nuclear translocation of phosphorylated IRF-3, association with the transcriptional coactivator CBP/p300, and stimulation of DNA binding and transcriptional activities of virus-inducible genes. Using yeast and mammalian one-hybrid analysis, we now demonstrate that an extended, atypical transactivation domain is located in the C terminus of IRF-3 between amino acids (aa) 134 and 394. We also show that the C-terminal domain of IRF-3 located between aa 380 and 427 participates in the autoinhibition of IRF-3 activity via an intramolecular association with the N-terminal region between aa 98 and 240. After Sendai virus infection, an intermolecular association between IRF-3 proteins is detected, demonstrating a virus-dependent formation of IRF-3 homodimers; this interaction is also observed in the absence of virus infection with a constitutively activated form of IRF-3. Substitution of the C-terminal Ser-Thr phosphorylation sites with the phosphomimetic Asp in the region ISNSHPLSLTSDQ between amino acids 395 and 407 [IRF-3(5D)], but not the adjacent S385 and S386 residues, generates a constitutively activated DNA binding form of IRF-3. In contrast, substitution of S385 and S386 with either Ala or Asp inhibits both DNA binding and transactivation activities of the IRF-3(5D) protein. These studies thus define the transactivation domain of IRF-3, two domains that participate in the autoinhibition of IRF-3 activity, and the regulatory phosphorylation sites controlling IRF-3 dimer formation, DNA binding activity, and association with the CBP/p300 coactivator.
Figures






Similar articles
-
Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation.Mol Cell Biol. 1998 May;18(5):2986-96. doi: 10.1128/MCB.18.5.2986. Mol Cell Biol. 1998. PMID: 9566918 Free PMC article.
-
Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300.EMBO J. 1998 Feb 16;17(4):1087-95. doi: 10.1093/emboj/17.4.1087. EMBO J. 1998. PMID: 9463386 Free PMC article.
-
Transcriptional activity of interferon regulatory factor (IRF)-3 depends on multiple protein-protein interactions.Eur J Biochem. 2002 Dec;269(24):6142-51. doi: 10.1046/j.1432-1033.2002.03330.x. Eur J Biochem. 2002. PMID: 12473110
-
Triggering the interferon response: the role of IRF-3 transcription factor.J Interferon Cytokine Res. 1999 Jan;19(1):1-13. doi: 10.1089/107999099314360. J Interferon Cytokine Res. 1999. PMID: 10048763 Review.
-
Control of IRF-3 activation by phosphorylation.J Interferon Cytokine Res. 2002 Jan;22(1):73-6. doi: 10.1089/107999002753452674. J Interferon Cytokine Res. 2002. PMID: 11846977 Review.
Cited by
-
TRAF-interacting protein (TRIP) negatively regulates IFN-β production and antiviral response by promoting proteasomal degradation of TANK-binding kinase 1.J Exp Med. 2012 Sep 24;209(10):1703-11. doi: 10.1084/jem.20120024. Epub 2012 Sep 3. J Exp Med. 2012. PMID: 22945920 Free PMC article.
-
A comprehensive map of the toll-like receptor signaling network.Mol Syst Biol. 2006;2:2006.0015. doi: 10.1038/msb4100057. Epub 2006 Apr 18. Mol Syst Biol. 2006. PMID: 16738560 Free PMC article. Review.
-
Inhibition of lipopolysaccharide-induced interferon regulatory factor 3 activation and protection from septic shock by hydroxystilbenes.Shock. 2004 May;21(5):470-5. doi: 10.1097/01.shk.0000123513.13212.83. Shock. 2004. PMID: 15087825 Free PMC article.
-
Downregulation of IRF-3 levels by ribozyme modulates the profile of IFNA subtypes expressed in infected human cells.J Virol. 2001 Mar;75(6):3021-7. doi: 10.1128/JVI.75.6.3021-3027.2001. J Virol. 2001. PMID: 11222729 Free PMC article.
-
High-Risk Mucosal Human Papillomavirus 16 (HPV16) E6 Protein and Cutaneous HPV5 and HPV8 E6 Proteins Employ Distinct Strategies To Interfere with Interferon Regulatory Factor 3-Mediated Beta Interferon Expression.J Virol. 2022 May 25;96(10):e0187521. doi: 10.1128/jvi.01875-21. Epub 2022 Apr 27. J Virol. 2022. PMID: 35475668 Free PMC article.
References
-
- Bovolenta C, Driggers P H, Marks M S, Medin J A, Politis A D, Vogel S N, Levy D E, Sakaguchi K, Appella E, Coligan J E, Ozato K. Molecular interactions between interferon consensus sequence binding protein and members of the interferon regulatory factor family. Proc Natl Acad Sci USA. 1994;91:5046–5050. - PMC - PubMed
-
- Bragança J, Génin P, Bandu M-T, Darracq N, Vignal M, Cassé C, Doly J, Civas A. Synergism between multiple virus-induced-factor-binding elements involved in the differential expression of IFN-A genes. J Biol Chem. 1997;272:22154–22162. - PubMed
-
- Brass A, Kehrli E, Eisenbeis C, Storb U, Singh H. Pip, a lymphoid restricted IRF, contains a regulatory domain that is important for autoinhibition and ternary complex formation with the Ets factor PU.1. Genes Dev. 1996;10:2335–2347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
