Synergism with germ line transcription factor Oct-4: viral oncoproteins share the ability to mimic a stem cell-specific activity
- PMID: 10082529
- PMCID: PMC84056
- DOI: 10.1128/MCB.19.4.2635
Synergism with germ line transcription factor Oct-4: viral oncoproteins share the ability to mimic a stem cell-specific activity
Abstract
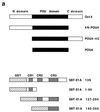
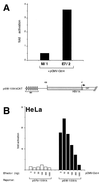
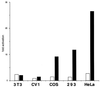
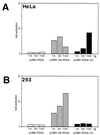
Activation of transcription by Oct-4 from remote binding sites requires a cofactor that is restricted to embryonal stem cells. The adenovirus E1A protein can mimic the activity of this stem cell-specific factor and stimulates Oct-4 activity in differentiated cells. Here we characterize the Oct-4-E1A interaction and show that the E1A 289R protein harbors two independent Oct-4 binding sites, both of which specifically interact with the POU domain of Oct-4. Furthermore, we demonstrate that, like E1A, the human papillomavirus E7 oncoprotein also specifically binds to the Oct-4 POU domain. E7 and Oct-4 can form a complex both in vitro and in vivo. Expression of E7 in differentiated cells stimulates Oct-4-mediated transactivation from distal binding sites. Moreover, Oct-4, but not other Oct factors, is active when expressed in cells transformed by human papillomavirus. Our results suggest that different viruses have evolved oncoproteins that share the ability to target Oct-4 and to mimic a stem cell-specific activity.
Figures



Similar articles
-
The human papillomavirus type 16 E7 protein complements adenovirus type 5 E1A amino-terminus-dependent transactivation of adenovirus type 5 early genes and increases ATF and Oct-1 DNA binding activity.J Virol. 1996 Jan;70(1):332-40. doi: 10.1128/JVI.70.1.332-340.1996. J Virol. 1996. PMID: 8523545 Free PMC article.
-
A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells.Cell. 1991 Jul 26;66(2):291-304. doi: 10.1016/0092-8674(91)90619-a. Cell. 1991. PMID: 1830243
-
Mutagenesis of the pRB pocket reveals that cell cycle arrest functions are separable from binding to viral oncoproteins.Mol Cell Biol. 2000 May;20(10):3715-27. doi: 10.1128/MCB.20.10.3715-3727.2000. Mol Cell Biol. 2000. PMID: 10779361 Free PMC article.
-
The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110.Oncogene. 1995 Nov 2;11(9):1859-64. Oncogene. 1995. PMID: 7478615
-
Biological activities and molecular targets of the human papillomavirus E7 oncoprotein.Oncogene. 2001 Nov 26;20(54):7888-98. doi: 10.1038/sj.onc.1204860. Oncogene. 2001. PMID: 11753671 Review.
Cited by
-
Clinicopathological significance of non-small cell lung cancer with high prevalence of Oct-4 tumor cells.J Exp Clin Cancer Res. 2012 Feb 2;31(1):10. doi: 10.1186/1756-9966-31-10. J Exp Clin Cancer Res. 2012. PMID: 22300949 Free PMC article.
-
HPV-Induced Field Cancerisation: Transformation of Adult Tissue Stem Cell Into Cancer Stem Cell.Front Microbiol. 2018 Mar 26;9:546. doi: 10.3389/fmicb.2018.00546. eCollection 2018. Front Microbiol. 2018. PMID: 29632522 Free PMC article.
-
Inflammation and Stem Cell Stochasticity of HPV-induced Cervical Cancer: Epigenetics based Biomarkers through Microbiome and Metabolome for Personalized Medicine: A Systematic Review.Curr Med Chem. 2025;32(12):2390-2408. doi: 10.2174/0109298673257429231108072717. Curr Med Chem. 2025. PMID: 38018189
-
Oct-4 controls cell-cycle progression of embryonic stem cells.Biochem J. 2010 Feb 9;426(2):171-81. doi: 10.1042/BJ20091439. Biochem J. 2010. PMID: 19968627 Free PMC article.
-
Phosphorylation of Threonine343 Is Crucial for OCT4 Interaction with SOX2 in the Maintenance of Mouse Embryonic Stem Cell Pluripotency.Stem Cell Reports. 2017 Nov 14;9(5):1630-1641. doi: 10.1016/j.stemcr.2017.09.001. Epub 2017 Oct 5. Stem Cell Reports. 2017. PMID: 28988986 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
