Functional domains of c-myc promoter binding protein 1 involved in transcriptional repression and cell growth regulation
- PMID: 10082554
- PMCID: PMC84081
- DOI: 10.1128/MCB.19.4.2880
Functional domains of c-myc promoter binding protein 1 involved in transcriptional repression and cell growth regulation
Abstract
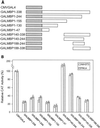
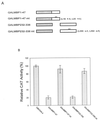
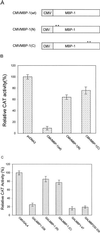
We initially identified c-myc promoter binding protein 1 (MBP-1), which negatively regulates c-myc promoter activity, from a human cervical carcinoma cell expression library. Subsequent studies on the biological role of MBP-1 demonstrated induction of cell death in fibroblasts and loss of anchorage-independent growth, reduced invasive ability, and tumorigenicity of human breast carcinoma cells. To investigate the potential role of MBP-1 as a transcriptional regulator, a chimeric protein containing MBP-1 fused to the DNA binding domain of the yeast transactivator factor GAL4 was constructed. This fusion protein exhibited repressor activity on the herpes simplex virus thymidine kinase promoter via upstream GAL4 DNA binding sites. Structure-function analysis of mutant MBP-1 in the context of the GAL4 DNA binding domain revealed that MBP-1 transcriptional repressor domains are located in the N terminus (amino acids 1 to 47) and C terminus (amino acids 232 to 338), whereas the activation domain lies in the middle (amino acids 140 to 244). The N-terminal domain exhibited stronger transcriptional repressor activity than the C-terminal region. When the N-terminal repressor domain was transferred to a potent activator, transcription was strongly inhibited. Both of the repressor domains contained hydrophobic regions and had an LXVXL motif in common. Site-directed mutagenesis in the repressor domains indicated that the leucine residues in the LXVXL motif are required for transcriptional repression. Mutation of the leucine residues in the common motif of MBP-1 also abrogated the repressor activity on the c-myc promoter. In addition, the leucine mutant forms of MBP-1 failed to suppress cell growth in fibroblasts like wild-type MBP-1. Taken together, our results indicate that MBP-1 is a complex cellular factor containing multiple transcriptional regulatory domains that play an important role in cell growth regulation.
Figures




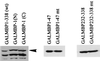
References
-
- Baichwal V R, Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990;63:815–825. - PubMed
-
- Bushmeyer S, Park K, Atchison M L. Characterization of functional domains within the multifunctional transcription factor YY1. J Biol Chem. 1995;270:30213–30220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
