Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis
- PMID: 10082577
- PMCID: PMC84104
- DOI: 10.1128/MCB.19.4.3103
Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis
Abstract
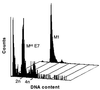
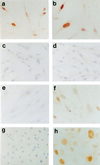
The accumulation of genetic abnormalities in a developing tumor is driven, at least in part, by the need to overcome inherent restraints on the replicative life span of human cells, two of which-senescence (M1) and crisis (M2)-have been well characterized. Here we describe additional barriers to clonal expansion (Mint) intermediate between M1 and M2, revealed by abrogation of tumor-suppressor gene (TSG) pathways by individual human papillomavirus type 16 (HPV16) proteins. In human fibroblasts, abrogation of p53 function by HPVE6 allowed escape from M1, followed up to 20 population doublings (PD) later by a second viable proliferation arrest state, MintE6, closely resembling M1. This occurred despite abrogation of p21(WAF1) induction but was associated with and potentially mediated by a further approximately 3-fold increase in p16(INK4a) expression compared to its level at M1. Expression of HPVE7, which targets pRb (and p21(WAF1)), also permitted clonal expansion, but this was limited predominantly by increasing cell death, resulting in a MintE7 phenotype similar to M2 but occurring after fewer PD. This was associated with, and at least partly due to, an increase in nuclear p53 content and activity, not seen in younger cells expressing E7. In a different cell type, thyroid epithelium, E7 also allowed clonal expansion terminating in a similar state to MintE7 in fibroblasts. In contrast, however, there was no evidence for a p53-regulated pathway; E6 was without effect, and the increases in p21(WAF1) expression at M1 and MintE7 were p53 independent. These data provide a model for clonal evolution by successive TSG inactivation and suggest that cell type diversity in life span regulation may determine the pattern of gene mutation in the corresponding tumors.
Figures







References
-
- Afshari C A, Vojta P J, Annab L A, Futreal P A, Willard T B, Barrett J C. Investigation of the role of G1/S cell cycle mediators in cellular senescence. Exp Cell Res. 1993;209:231–237. - PubMed
-
- Bacchetti S. Telomere dynamics and telomerase activity in cell senescence and cancer. Cell Dev Biol. 1996;7:31–39.
-
- Banks L, Matlashewski G, Crawford L. Isolation of human p53 specific monoclonal antibodies and their use in the study of human p53 expression. Eur J Biochem. 1986;159:529–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
