Chemokine secretion of human cells in response to Toxoplasma gondii infection
- PMID: 10084985
- PMCID: PMC96495
- DOI: 10.1128/IAI.67.4.1547-1552.1999
Chemokine secretion of human cells in response to Toxoplasma gondii infection
Abstract
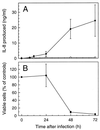
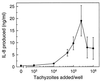
The ubiquitous protozoan parasite Toxoplasma gondii is a major cause of morbidity and mortality in neonates and immunocompromised hosts. Both acute invasion and reactivation of latent infection result in an inflammatory reaction with lymphocytes, macrophages, and neutrophils. The mechanisms responsible for triggering the local host response to toxoplasmosis are not fully understood. Infection of monolayers of human HeLa epithelial cells and fibroblasts with T. gondii resulted in a marked increase in the expression of interleukin-8 (IL-8)-specific mRNA and secretion of the proinflammatory and chemoattractant cytokines interleukin-8 (IL-8), GROalpha, and MCP-1. Host cell invasion and lysis were required for this response, as tachyzoite lysates alone had no effect on IL-8 secretion. IL-8 release was dependent on the release of soluble host cell factors: IL-1alpha in HeLa cells and an additional mediator in fibroblasts. HT-29 epithelial cells, which lack IL-1alpha or another IL-8-inducing activity, did not release IL-8 after infection, although they were efficiently infected with T. gondii and increased IL-8 secretion in response to added IL-1alpha. These data suggest that proinflammatory chemokine secretion is an important host cell response to toxoplasmosis and that the release of IL-1alpha and other mediators from lysed host cells is critical for this chemokine response.
Figures


Similar articles
-
Nuclear factor-kappa B plays a major role in the regulation of chemokine expression of HeLa cells in response to Toxoplasma gondii infection.Parasitol Res. 2001 Sep;87(9):758-63. doi: 10.1007/s004360100447. Parasitol Res. 2001. PMID: 11570562
-
Evasion of Human Neutrophil-Mediated Host Defense during Toxoplasma gondii Infection.mBio. 2018 Feb 13;9(1):e02027-17. doi: 10.1128/mBio.02027-17. mBio. 2018. PMID: 29440572 Free PMC article.
-
Infection of human astrocytes and glioblastoma cells with Toxoplasma gondii: monocyte chemotactic protein-1 secretion and chemokine expression in vitro.Acta Neuropathol. 2004 Mar;107(3):245-9. doi: 10.1007/s00401-003-0804-0. Epub 2004 Jan 14. Acta Neuropathol. 2004. PMID: 14722715
-
From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii.FEMS Immunol Med Microbiol. 2003 Dec 5;39(3):193-203. doi: 10.1016/S0928-8244(03)00279-7. FEMS Immunol Med Microbiol. 2003. PMID: 14642303 Review.
-
A review: Competence, compromise, and concomitance-reaction of the host cell to Toxoplasma gondii infection and development.J Parasitol. 2011 Aug;97(4):620-8. doi: 10.1645/GE-2712.1. Epub 2011 Feb 11. J Parasitol. 2011. PMID: 21506833 Review.
Cited by
-
Differential interleukin-8 and nitric oxide production in epithelial cells induced by mucosally invasive and noninvasive Trypanosoma cruzi trypomastigotes.Infect Immun. 2003 Sep;71(9):5394-7. doi: 10.1128/IAI.71.9.5394-5397.2003. Infect Immun. 2003. PMID: 12933891 Free PMC article.
-
Immune Mediator Profile in Aqueous Humor Differs in Patients with Primary Acquired Ocular Toxoplasmosis and Recurrent Acute Ocular Toxoplasmosis.Mediators Inflamm. 2019 Feb 17;2019:9356728. doi: 10.1155/2019/9356728. eCollection 2019. Mediators Inflamm. 2019. PMID: 30906227 Free PMC article.
-
Innate recognition of Toxoplasma gondii in humans involves a mechanism distinct from that utilized by rodents.Cell Mol Immunol. 2017 Jan;14(1):36-42. doi: 10.1038/cmi.2016.12. Epub 2016 May 9. Cell Mol Immunol. 2017. PMID: 27157497 Free PMC article. Review.
-
Toxoplasma gondii induces the secretion of monocyte chemotactic protein-1 in human fibroblasts, in vitro.Mol Cell Biochem. 2000 Jun;209(1-2):79-87. doi: 10.1023/a:1007075701551. Mol Cell Biochem. 2000. PMID: 10942204
-
cDNA microarray analysis of host-pathogen interactions in a porcine in vitro model for Toxoplasma gondii infection.Infect Immun. 2006 Jul;74(7):4254-65. doi: 10.1128/IAI.00386-05. Infect Immun. 2006. PMID: 16790800 Free PMC article.
References
-
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Bertoli F, Espino M, Arosemena J R, Fishback J L, Frenkel J K. A spectrum in the pathology of toxoplasmosis in patients with AIDS. Arch Pathol Lab Med. 1995;119:214–224. - PubMed
-
- Carrazana E J, Rossitch E, Samuels M A. Cerebral toxoplasmosis in the acquired immune deficiency syndrome. Clin Neurol Neurosurg. 1989;91:291–299. - PubMed
-
- Dupey J P, Speer C A, Shen S K, Kwok O C H, Blixt J A. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J Parasitol. 1997;83:870–882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

