NF-kappaB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression
- PMID: 10084986
- PMCID: PMC96496
- DOI: 10.1128/IAI.67.4.1553-1559.1999
NF-kappaB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression
Abstract
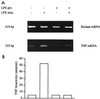
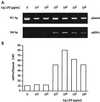
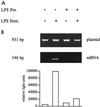
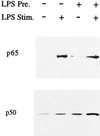
Monocytes respond to lipopolysaccharide (LPS) stimulation with a rapid expression of the tumor necrosis factor (TNF) gene. Upon repeated LPS stimulation there is, however, little production of TNF mRNA and protein; i.e., the cells are tolerant to LPS. Analysis of NF-kappaB proteins in gel shift assays demonstrated that the DNA binding activity that is induced by LPS stimulation in tolerant cells consists mainly of p50-p50 homodimers. Since p50 can bind to DNA but lacks a transactivation domain, this may explain the blockade of TNF gene expression. We now show that in the monocytic cell line Mono Mac 6, this inability to respond can be largely ascribed to NF-kappaB, since a reporter construct directed by a trimeric NF-kappaB motif is strongly transactivated by LPS stimulation of naive cells whereas LPS-tolerant cells exhibit only low activity. Also, Western blot analyses of proteins extracted from purified nuclei showed mobilization of threefold-higher levels of p50 protein in tolerant compared to naive cells, while mobilization of p65 was unaltered. Overexpression of p50 in HEK 293 cells resulted in a strong reduction of p65-driven TNF promoter activity at the levels of both luciferase mRNA and protein. These data support the concept that an upregulation of p50 is instrumental in LPS tolerance in human monocytes.
Figures

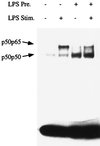

References
-
- Blackwell T S, Blackwell T R, Christman J W. Induction of endotoxin tolerance depletes nuclear factor-κB and suppresses its activation in rat alveolar macrophages. J Leukoc Biol. 1997;62:885–891. - PubMed
-
- Coffee K A, Halushka P V, Ashton S H, Tempel G E, Wise W C, Cook J A. Endotoxin tolerance is associated with altered GTP-binding protein function. J Appl Physiol. 1992;73:1008–1013. - PubMed
-
- Durando M, Ashton S H, Makhlouf M A, Simmons-Wagner R, Halushka P V, Cook J A. Endotoxin-induced desensitization of THP-1 cells is not associated with altered G protein binding or content. J Endotoxin Res. 1997;4:97–103.
-
- Ertel W, Kremer J-P, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg F W. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

