Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice
- PMID: 10085018
- PMCID: PMC96528
- DOI: 10.1128/IAI.67.4.1779-1788.1999
Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice
Abstract
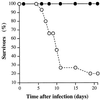
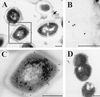
The role of an extracellular cysteine protease encoded by the speB gene in group A Streptococcus (GAS) skin infection was studied with a mouse model. Mice were injected subcutaneously with a wild-type GAS serotype M3 strain or a cysteine protease-inactivated isogenic derivative grown to stationary phase. The mortality rate of mice injected with the M3 speB mutant strain was significantly decreased (P < 0.0008) compared to that of animals injected with the wild-type parental organism. The abscesses formed in animals infected with the cysteine protease mutant strain were significantly smaller (P < 0.0001) than those caused by the wild-type organism and slowly regressed over 3 to 4 weeks. In striking contrast, infection with the wild-type GAS isolate generated necrotic lesions, and in some animals the GAS disseminated widely from the injection site and produced extensive cutaneous damage. All of these animals developed bacteremia and died. GAS dissemination was accompanied by severe tissue and blood vessel necrosis. Cysteine protease expression in the infected tissue was identified by immunogold electron microscopy. These data demonstrate that cysteine protease expression contributes to soft tissue pathology, including necrosis, and is required for efficient systemic dissemination of the organism from the initial site of skin inoculation.
Figures
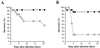


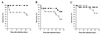
References
-
- Belani K, Schlievert P M, Kaplan E L, Ferrieri P. Association of exotoxin-producing group A streptococci and severe disease in children. Pediatr Infect Dis J. 1991;10:351–354. - PubMed
-
- Boyle M D P, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. - PubMed
-
- Burns E H, Jr, Lukomski S, Rurangirwa J, Podbielski A, Musser J M. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb Pathog. 1998;24:333–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

