Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity
- PMID: 10085030
- PMCID: PMC96540
- DOI: 10.1128/IAI.67.4.1871-1877.1999
Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity
Abstract
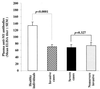
An impressive change in the epidemiology and severity of invasive group A streptococcal infections occurred in the 1980s, and the incidence of streptococcal toxic shock syndrome cases continues to rise. The reason for the resurgence of severe invasive cases remains a mystery-has there been a change in the pathogen or in host protective immunity? To address these questions, we have studied 33 patients with invasive infection caused by genotypically indistinguishable M1T1 strains of Streptococcus pyogenes who had different disease outcomes. Patients were classified as having severe (n = 21) and nonsevere (n = 12) invasive infections based on the presence or absence of shock and organ failure. Levels of anti-M1 bactericidal antibodies and of anti-streptococcal superantigen neutralizing antibodies in plasma were significantly lower in both groups than in age- and geographically matched healthy controls (P < 0.01). Importantly, the levels of these protective antibodies in plasma samples from severe and nonsevere invasive cases were not different. Together the data suggest that low levels of protective antibodies may contribute to host susceptibility to invasive streptococcal infection but do not modulate disease outcome. Other immunogenetic factors that regulate superantigen responses may influence the severity of systemic manifestations associated with invasive streptococcal infection.
Figures


References
-
- Alouf J E, Knoll H, Kohler W. The family of mitogenic, shock-inducing and superantigenic toxins from staphylococci and streptococci. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. pp. 367–414.
-
- Basma H, Norrby-Teglund A, Low D E, McGeer A, El-Ahmady O, Kotb M. Opsonic antibodies to the surface M protein of group A streptococci in pooled normal immunoglobulin G (IVIG): potential impact on the clinical efficacy of IVIG in severe invasive group A streptococcal infections. Infect Immun. 1998;66:2279–2283. - PMC - PubMed
-
- Beachey E H, Seyer J M. Primary structure and immunochemistry of group A streptococcal M proteins. Semin Infect Dis. 1982;4:401–410.
-
- Bisno A, Stevens D. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. - PubMed
-
- Campbell J R, Arango C A, Garcia-Prats J A, Baker C J. An outbreak of M serotype 1 group A streptococcus in a neonatal intensive care unit. J Pediatr. 1996;129:396–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

