Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis
- PMID: 10085289
- PMCID: PMC2148190
- DOI: 10.1083/jcb.144.5.891
Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis
Abstract
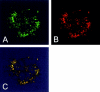
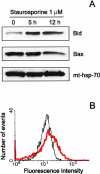
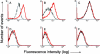
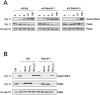
Here we report that in staurosporine-induced apoptosis of HeLa cells, Bid, a BH3 domain containing protein, translocates from the cytosol to mitochondria. This event is associated with a change in conformation of Bax which leads to the unmasking of its NH2-terminal domain and is accompanied by the release of cytochrome c from mitochondria. A similar finding is reported for cerebellar granule cells undergoing apoptosis induced by serum and potassium deprivation. The Bax-conformational change is prevented by Bcl-2 and Bcl-xL but not by caspase inhibitors. Using isolated mitochondria and various BH3 mutants of Bid, we demonstrate that direct binding of Bid to Bax is a prerequisite for Bax structural change and cytochrome c release. Bcl-xL can inhibit the effect of Bid by interacting directly with Bax. Moreover, using mitochondria from Bax-deficient tumor cell lines, we show that Bid- induced release of cytochrome c is negligible when Bid is added alone, but dramatically increased when Bid and Bax are added together. Taken together, our results suggest that, during certain types of apoptosis, Bid translocates to mitochondria and binds to Bax, leading to a change in conformation of Bax and to cytochrome c release from mitochondria.
Figures




References
-
- Antonsson B, Conti F, Ciavatta AM, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. - PubMed
-
- Aritomi M, Kunishima N, Inohara N, Ishibashi Y, Ohta S, Morikawa K. Crystal structure of rat Bcl-xL . J Biol Chem. 1997;272:27886–27892. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Cheng EH-Y, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-xL . Nature. 1996;379:554–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

