Microtubule dynamics from mating through the first zygotic division in the budding yeast Saccharomyces cerevisiae
- PMID: 10085295
- PMCID: PMC2148199
- DOI: 10.1083/jcb.144.5.977
Microtubule dynamics from mating through the first zygotic division in the budding yeast Saccharomyces cerevisiae
Abstract
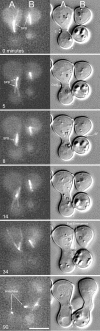
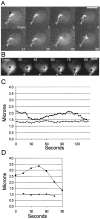
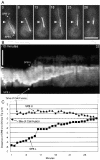
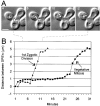
We have used time-lapse digital imaging microscopy to examine cytoplasmic astral microtubules (Mts) and spindle dynamics during the mating pathway in budding yeast Saccharomyces cerevisiae. Mating begins when two cells of opposite mating type come into proximity. The cells arrest in the G1 phase of the cell cycle and grow a projection towards one another forming a shmoo projection. Imaging of microtubule dynamics with green fluorescent protein (GFP) fusions to dynein or tubulin revealed that the nucleus and spindle pole body (SPB) became oriented and tethered to the shmoo tip by a Mt-dependent search and capture mechanism. Dynamically unstable astral Mts were captured at the shmoo tip forming a bundle of three or four astral Mts. This bundle changed length as the tethered nucleus and SPB oscillated toward and away from the shmoo tip at growth and shortening velocities typical of free plus end astral Mts (approximately 0.5 micrometer/min). Fluorescent fiduciary marks in Mt bundles showed that Mt growth and shortening occurred primarily at the shmoo tip, not the SPB. This indicates that Mt plus end assembly/disassembly was coupled to pushing and pulling of the nucleus. Upon cell fusion, a fluorescent bar of Mts was formed between the two shmoo tip bundles, which slowly shortened (0.23 +/- 0.07 micrometer/min) as the two nuclei and their SPBs came together and fused (karyogamy). Bud emergence occurred adjacent to the fused SPB approximately 30 min after SPB fusion. During the first mitosis, the SPBs separated as the spindle elongated at a constant velocity (0.75 micrometer/min) into the zygotic bud. There was no indication of a temporal delay at the 2-micrometer stage of spindle morphogenesis or a lag in Mt nucleation by replicated SPBs as occurs in vegetative mitosis implying a lack of normal checkpoints. Thus, the shmoo tip appears to be a new model system for studying Mt plus end dynamic attachments and much like higher eukaryotes, the first mitosis after haploid cell fusion in budding yeast may forgo cell cycle checkpoints present in vegetative mitosis.
Figures






Similar articles
-
Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud.J Cell Biol. 1997 Nov 17;139(4):985-94. doi: 10.1083/jcb.139.4.985. J Cell Biol. 1997. PMID: 9362516 Free PMC article.
-
The minus end-directed motor Kar3 is required for coupling dynamic microtubule plus ends to the cortical shmoo tip in budding yeast.Curr Biol. 2003 Aug 19;13(16):1423-8. doi: 10.1016/s0960-9822(03)00547-5. Curr Biol. 2003. PMID: 12932327
-
Collective dynein transport of the nucleus by pulling on astral microtubules during Saccharomyces cerevisiae mitosis.Yeast. 2021 Jun;38(6):352-366. doi: 10.1002/yea.3552. Epub 2021 Feb 27. Yeast. 2021. PMID: 33547820
-
Microtubule dynamics in the budding yeast mating pathway.J Cell Sci. 2006 Sep 1;119(Pt 17):3485-90. doi: 10.1242/jcs.03193. J Cell Sci. 2006. PMID: 16931596 Review.
-
Life cycles of yeast spindle pole bodies: getting microtubules into a closed nucleus.Biol Cell. 1999 May-Jun;91(4-5):305-12. Biol Cell. 1999. PMID: 10518997 Review.
Cited by
-
Vik1 modulates microtubule-Kar3 interactions through a motor domain that lacks an active site.Cell. 2007 Mar 23;128(6):1161-72. doi: 10.1016/j.cell.2006.12.046. Cell. 2007. PMID: 17382884 Free PMC article.
-
The protease activity of yeast separase (esp1) is required for anaphase spindle elongation independently of its role in cleavage of cohesin.Genetics. 2008 Apr;178(4):2361-72. doi: 10.1534/genetics.107.085308. Genetics. 2008. PMID: 18430955 Free PMC article.
-
Improved Plasmids for Fluorescent Protein Tagging of Microtubules in Saccharomyces cerevisiae.Traffic. 2015 Jul;16(7):773-786. doi: 10.1111/tra.12276. Epub 2015 Apr 28. Traffic. 2015. PMID: 25711127 Free PMC article.
-
Molecular basis of Kar9-Bim1 complex function during mating and spindle positioning.Mol Biol Cell. 2016 Sep 28;27(23):3729-45. doi: 10.1091/mbc.E16-07-0552. Online ahead of print. Mol Biol Cell. 2016. PMID: 27682587 Free PMC article.
-
The mating-specific Galpha interacts with a kinesin-14 and regulates pheromone-induced nuclear migration in budding yeast.Mol Biol Cell. 2009 Jun;20(12):2820-30. doi: 10.1091/mbc.e09-01-0069. Epub 2009 Apr 22. Mol Biol Cell. 2009. PMID: 19386762 Free PMC article.
References
-
- Brinkley RR. Microtubule organizing centers. Ann Rev Cell Biol. 1985;1:145–172. - PubMed
-
- Byers, B. 1981. Cytology of the yeast life cycle. In The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. J. Strachern, E.W. Jones, and J.R. Broach, eds. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 59–96.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

