Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts
- PMID: 10085298
- PMCID: PMC2148201
- DOI: 10.1083/jcb.144.5.1019
Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts
Abstract
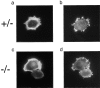
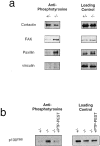
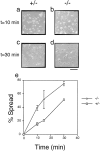
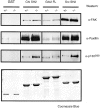
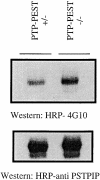
In this article, we show that, in transfected COS-1 cells, protein tyrosine phosphatase (PTP)-PEST translocates to the membrane periphery following stimulation by the extracellular matrix protein fibronectin. When plated on fibronectin, PTP-PEST (-/-) fibroblasts display a strong defect in motility. 3 h after plating on fibronectin, the number and size of vinculin containing focal adhesions were greatly increased in the homozygous PTP-PEST mutant cells as compared with heterozygous cells. This phenomenon appears to be due in part to a constitutive increase in tyrosine phosphorylation of p130(CAS), a known PTP-PEST substrate, paxillin, which associates with PTP-PEST in vitro, and focal adhesion kinase (FAK). Another effect of this constitutive hyperphosphorylation, consistent with the focal adhesion regulation defect, is that (-/-) cells spread faster than the control cell line when plated on fibronectin. In the PTP-PEST (-/-) cells, an increase in affinity for the SH2 domains of Src and Crk towards p130(CAS) was also observed. In (-/-) cells, we found a significant increase in the level of tyrosine phosphorylation of PSTPIP, a cleavage furrow-associated protein that interacts physically with all PEST family members. An effect of PSTPIP hyperphosphorylation appears to be that some cells remain attached at the site of the cleavage furrow for an extended period of time. In conclusion, our data suggest PTP-PEST plays a dual role in cell cytoskeleton organization, by promoting the turnover of focal adhesions required for cell migration, and by directly or indirectly regulating the proline, serine, threonine phosphatase interacting protein (PSTPIP) tyrosine phosphorylation level which may be involved in regulating cleavage furrow formation or disassembly during normal cell division.
Figures















References
-
- Akasaka T, van Leeuwen RL, Yoshinaga IG, Mihm MC, Jr, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Investig Dermatol. 1995;105:104–108. - PubMed
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

