Topographic organization of human visual areas in the absence of input from primary cortex
- PMID: 10087075
- PMCID: PMC6786078
- DOI: 10.1523/JNEUROSCI.19-07-02619.1999
Topographic organization of human visual areas in the absence of input from primary cortex
Abstract
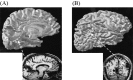
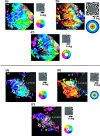
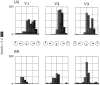
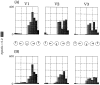
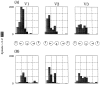
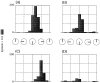
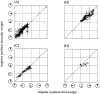
Recently, there has been evidence for considerable plasticity in primary sensory areas of adult cortex. In this study, we asked to what extent topographical maps in human extrastriate areas reorganize after damage to a portion of primary visual (striate) cortex, V1. Functional magnetic resonance imaging signals were measured in a subject (G.Y.) with a large calcarine lesion that includes most of primary visual cortex but spares the foveal representation. When foveal stimulation was present, intact cortex in the lesioned occipital lobe exhibited conventional retinotopic organization. Several visual areas could be identified (V1, V2, V3, V3 accessory, and V4 ventral). However, when stimuli were restricted to the blind portion of the visual field, responses were found primarily in dorsal extrastriate areas. Furthermore, cortex that had formerly shown normal topography now represented only the visual field around the lower vertical meridian. Several possible sources for this reorganized activity are considered, including transcallosal connections, direct subcortical projections to extrastriate cortex, and residual inputs from V1 near the margin of the lesion. A scheme is described to explain how the reorganized signals could occur based on changes in the local neural connections.
Figures


References
-
- Barbur JL, Ruddock KH. Spatial characteristics of movement detection mechanisms in human vision. I. Achromatic vision. Biol Cybern. 1980;37:77–92. - PubMed
-
- Barbur JL, Ruddock KH, Waterfield VA. Human visual responses in the absence of the geniculo-calcarine projection. Brain. 1980;103:905–928. - PubMed
-
- Barbur JL, Watson JDG, Frackowiak RSJ, Zeki S. Conscious visual perception without V1. Brain. 1993;116:1293–1302. - PubMed
-
- Berson DM, Stein JJ. Retinotopic organization of the superior colliculus in relation to the retinal distribution of afferent ganglion cells. Vis Neurosci. 1995;12:671–686. - PubMed
-
- Blythe IM, Kennard C, Ruddock KH. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain. 1987;110:887–905. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
