Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane
- PMID: 10087079
- PMCID: PMC6786082
- DOI: 10.1523/JNEUROSCI.19-07-02658.1999
Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane
Abstract
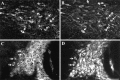
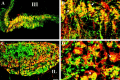
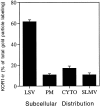
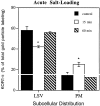
We examined the cellular and subcellular distribution of the cloned kappa opioid receptor (KOR1) and its trafficking to the presynaptic plasma membrane in vasopressin magnocellular neurosecretory neurons. We used immunohistochemistry to show that KOR1 immunoreactivity (IR) colocalized with vasopressin-containing cell bodies, axons, and axon terminals within the posterior pituitary. Ultrastructural analysis revealed that a major fraction of KOR1-IR was associated with the membrane of peptide-containing large secretory vesicles. KOR1-IR was rarely associated with the plasma membrane in unstimulated nerve terminals within the posterior pituitary. A physiological stimulus (salt-loading) that elicits vasopressin release also caused KOR1-IR to translocate from these vesicles to the plasma membrane. After stimulation, there was a significant decrease in KOR1-IR associated with peptide-containing vesicles and a significant increase in KOR1-IR associated with the plasma membrane. This stimulus-dependent translocation of receptors to the presynaptic plasma membrane provides a novel mechanism for regulation of transmitter release.
Figures


References
-
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–1463. - PubMed
-
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee J-H, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R. The κ-opioid receptor (KOR1) is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995;92:5062–5066. - PMC - PubMed
-
- Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem. 1994;42:1635–1642. - PubMed
-
- Boersma CJC, Sonnemans MA, Van Leeuwen FW. Immunoelectron microscopic demonstration of oxytocin and vasopressin in pituicytes and in nerve terminals forming synaptoid contacts with pituicytes in the rat neural lobe. Brain Res. 1993;611:117–129. - PubMed
-
- Boersma CJC, Pool CW, Van Heerikhuize J, Van Leeuwen FW. Characterization of opioid binding sites in the neural and intermediate lobe of the rat pituitary gland by quantitative receptor autoradiography. J Neuroendocrinol. 1994;6:47–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
