Reorganization of cholinergic terminals in the cerebral cortex and hippocampus in transgenic mice carrying mutated presenilin-1 and amyloid precursor protein transgenes
- PMID: 10087083
- PMCID: PMC6786057
- DOI: 10.1523/JNEUROSCI.19-07-02706.1999
Reorganization of cholinergic terminals in the cerebral cortex and hippocampus in transgenic mice carrying mutated presenilin-1 and amyloid precursor protein transgenes
Abstract
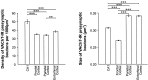
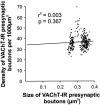
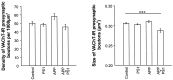
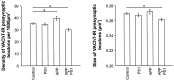
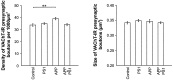
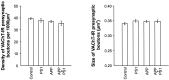
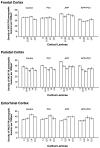
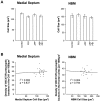
Cholinergic deficits are one of the most consistent neuropathological landmarks in Alzheimer's disease (AD). We have examined transgenic mouse models (PS1M146L, APPK670N,M671L) and a doubly transgenic line (APPK670N,M671L + PS1M146L) that overexpress mutated AD-related genes [presenilin-1 (PS1) and the amyloid precursor protein (APP)] to investigate the effect of AD-related gene overexpression and/or amyloidosis on cholinergic parameters. The size of the basal forebrain cholinergic neurons and the pattern of cholinergic synapses in the hippocampus and cerebral cortex were revealed by immunohistochemical staining for choline acetyltransferase and the vesicular acetylcholine transporter, respectively. At the time point studied (8 months), no apparent changes in either the size or density of cholinergic synapses were found in the PS1M146L mutant relative to the nontransgenic controls. However, the APPK670N,M671L mutant showed a significant elevation in the density of cholinergic synapses in the frontal and parietal cortices. Most importantly, the double mutant (APPK670N,M671L + PS1M146L), which had extensive amyloidosis, demonstrated a prominent diminution in the density of cholinergic synapses in the frontal cortex and a reduction in the size of these synapses in the frontal cortex and hippocampus. Nonetheless, no significant changes in the size of basal forebrain cholinergic neurons were observed in these three mutants. This study shows a novel role of APP and a synergistic effect of APP and PS1 that correlates with amyloid load on the reorganization of the cholinergic network in the cerebral cortex and hippocampus at the time point studied.
Figures



References
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
-
- Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. - PubMed
-
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand [Suppl] 1996;165:3–12. - PubMed
-
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
