A neurocomputational theory of the dopaminergic modulation of working memory functions
- PMID: 10087092
- PMCID: PMC6786084
- DOI: 10.1523/JNEUROSCI.19-07-02807.1999
A neurocomputational theory of the dopaminergic modulation of working memory functions
Abstract
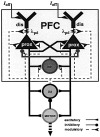
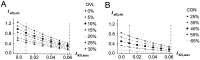
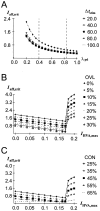
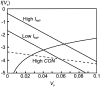
The dopaminergic modulation of neural activity in the prefrontal cortex (PFC) is essential for working memory. Delay-activity in the PFC in working memory tasks persists even if interfering stimuli intervene between the presentation of the sample and the target stimulus. Here, the hypothesis is put forward that the functional role of dopamine in working memory processing is to stabilize active neural representations in the PFC network and thereby to protect goal-related delay-activity against interfering stimuli. To test this hypothesis, we examined the reported dopamine-induced changes in several biophysical properties of PFC neurons to determine whether they could fulfill this function. An attractor network model consisting of model neurons was devised in which the empirically observed effects of dopamine on synaptic and voltage-gated membrane conductances could be represented in a biophysically realistic manner. In the model, the dopamine-induced enhancement of the persistent Na+ and reduction of the slowly inactivating K+ current increased firing of the delay-active neurons, thereby increasing inhibitory feedback and thus reducing activity of the "background" neurons. Furthermore, the dopamine-induced reduction of EPSP sizes and a dendritic Ca2+ current diminished the impact of intervening stimuli on current network activity. In this manner, dopaminergic effects indeed acted to stabilize current delay-activity. Working memory deficits observed after supranormal D1-receptor stimulation could also be explained within this framework. Thus, the model offers a mechanistic explanation for the behavioral deficits observed after blockade or after supranormal stimulation of dopamine receptors in the PFC and, in addition, makes some specific empirical predictions.
Figures





References
-
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. - PubMed
-
- Amit DJ. The Hebbian paradigm reintegrated: local reverberations as internal representations. Behav Brain Sci. 1995;18:617–657.
-
- Amit DJ, Brunel N. Learning internal representations in an attractor neural network with analogue neurons. Network. 1995;6:359–388.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
