Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source
- PMID: 10087257
- PMCID: PMC2150577
- DOI: 10.1083/jcb.144.6.1113
Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source
Abstract
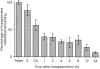
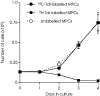
Myoblasts, the precursors of skeletal muscle fibers, can be induced to withdraw from the cell cycle and differentiate in vitro. Recent studies have also identified undifferentiated subpopulations that can self-renew and generate myogenic cells (Baroffio, A., M. Hamann, L. Bernheim, M.-L. Bochaton-Pillat, G. Gabbiani, and C.R. Bader. 1996. Differentiation. 60:47-57; Yoshida, N., S. Yoshida, K. Koishi, K. Masuda, and Y. Nabeshima. 1998. J. Cell Sci. 111:769-779). Cultured myoblasts can also differentiate and contribute to repair and new muscle formation in vivo, a capacity exploited in attempts to develop myoblast transplantation (MT) for genetic modification of adult muscle. Our studies of the dynamics of MT demonstrate that cultures of myoblasts contain distinct subpopulations defined by their behavior in vitro and divergent responses to grafting. By comparing a genomic and a semiconserved marker, we have followed the fate of myoblasts transplanted into muscles of dystrophic mice, finding that the majority of the grafted cells quickly die and only a minority are responsible for new muscle formation. This minority is behaviorally distinct, slowly dividing in tissue culture, but rapidly proliferative after grafting, suggesting a subpopulation with stem cell-like characteristics.
Figures




References
-
- Baroffio A, Bochaton-Piallet M-L, Gabbiani G, Bader CR. Heterogeneity in the progeny of single human muscle satellite cells. Differentiation. 1995;59:259–268. - PubMed
-
- Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat M-L, Gabbiani G, Bader CR. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. - PubMed
-
- Barr E, Leiden JM. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991;254:1507–1509. - PubMed
-
- Beauchamp JR, Pagel CN, Partridge TA. A dual-marker system for quantitative studies of myoblast transplantation in the mouse. Transplantation. 1997;63:1794–1797. - PubMed
-
- Blau HM, Springer ML. Muscle-mediated gene therapy. N Engl J Med. 1995;333:1554–1556. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

