minifly, a Drosophila gene required for ribosome biogenesis
- PMID: 10087258
- PMCID: PMC2150573
- DOI: 10.1083/jcb.144.6.1123
minifly, a Drosophila gene required for ribosome biogenesis
Abstract
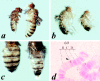
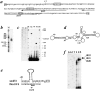
We report here the genetic, molecular, and functional characterization of the Drosophila melanogaster minifly (mfl) gene. Genetic analysis shows that mfl is essential for Drosophila viability and fertility. While P-element induced total loss-of-function mutations cause lethality, mfl partial loss-of-function mutations cause pleiotropic defects, such as extreme reduction of body size, developmental delay, hatched abdominal cuticle, and reduced female fertility. Morphological abnormalities characteristic of apoptosis are found in the ovaries, and a proportion of eggs laid by mfl mutant females degenerates during embryogenesis. We show that mfl encodes an ubiquitous nucleolar protein that plays a central role in ribosomal RNA processing and pseudouridylation, whose known eukaryotic homologues are yeast Cfb5p, rat NAP57 and human dyskerin, encoded by the gene responsible for the X-linked dyskeratosis congenita disease. mfl genetic analysis represents the first in vivo functional characterization of a member of this highly conserved gene family from higher eukaryotes. In addition, we report that mfl hosts an intron encoded box H/ACA snoRNA gene, the first member of this class of snoRNAs identified so far from Drosophila.
Figures





References
-
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophilaoogenesis. Development. 1993;117:29–43. - PubMed
-
- Ashburner, M. 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press. 434 pp.
-
- Bachellerie JP, Cavaille J. Guiding ribose methylation of rRNA. Trends Biochem Sci. 1997;22:257–261. - PubMed
-
- Bakin A, Ofengand J. Four newly located pseudouridylate residues in Escherichia coli23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993;32:9754–9762. - PubMed
-
- Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

