Rho GTPases control polarity, protrusion, and adhesion during cell movement
- PMID: 10087266
- PMCID: PMC2150589
- DOI: 10.1083/jcb.144.6.1235
Rho GTPases control polarity, protrusion, and adhesion during cell movement
Abstract
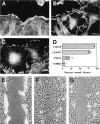
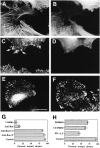
Cell movement is essential during embryogenesis to establish tissue patterns and to drive morphogenetic pathways and in the adult for tissue repair and to direct cells to sites of infection. Animal cells move by crawling and the driving force is derived primarily from the coordinated assembly and disassembly of actin filaments. The small GTPases, Rho, Rac, and Cdc42, regulate the organization of actin filaments and we have analyzed their contributions to the movement of primary embryo fibroblasts in an in vitro wound healing assay. Rac is essential for the protrusion of lamellipodia and for forward movement. Cdc42 is required to maintain cell polarity, which includes the localization of lamellipodial activity to the leading edge and the reorientation of the Golgi apparatus in the direction of movement. Rho is required to maintain cell adhesion during movement, but stress fibers and focal adhesions are not required. Finally, Ras regulates focal adhesion and stress fiber turnover and this is essential for cell movement. We conclude that the signal transduction pathways controlled by the four small GTPases, Rho, Rac, Cdc42, and Ras, cooperate to promote cell movement.
Figures


References
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Mastuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Anand-Apte B, Zetter BR, Viswanathan A, Qui R, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

