Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes
- PMID: 10087267
- PMCID: PMC2150578
- DOI: 10.1083/jcb.144.6.1245
Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes
Abstract
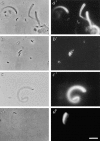
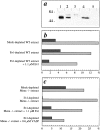
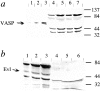
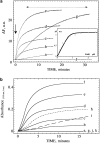
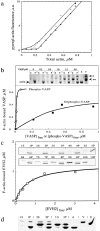
Intracellular propulsion of Listeria monocytogenes is the best understood form of motility dependent on actin polymerization. We have used in vitro motility assays of Listeria in platelet and brain extracts to elucidate the function of the focal adhesion proteins of the Ena (Drosophila Enabled)/VASP (vasodilator-stimulated phosphoprotein) family in actin-based motility. Immunodepletion of VASP from platelet extracts and of Evl (Ena/VASP-like protein) from brain extracts of Mena knockout (-/-) mice combined with add-back of recombinant (bacterial or eukaryotic) VASP and Evl show that VASP, Mena, and Evl play interchangeable roles and are required to transform actin polymerization into active movement and propulsive force. The EVH1 (Ena/VASP homology 1) domain of VASP is in slow association-dissociation equilibrium high-affinity binding to the zyxin-homologous, proline-rich region of ActA. VASP also interacts with F-actin via its COOH-terminal EVH2 domain. Hence VASP/ Ena/Evl link the bacterium to the actin tail, which is required for movement. The affinity of VASP for F-actin is controlled by phosphorylation of serine 157 by cAMP-dependent protein kinase. Phospho-VASP binds with high affinity (0.5 x 10(8) M-1); dephospho-VASP binds 40-fold less tightly. We propose a molecular ratchet model for insertional polymerization of actin, within which frequent attachment-detachment of VASP to F-actin allows its sliding along the growing filament.
Figures




References
-
- Abel, K., A. Lingnau, K. Niebuhr, J. Wehland, and U. Walter. 1996. Monoclonal antibodies against the focal adhesion protein VASP revealing epitopes involved in the interaction with two VASP binding proteins and VASP phosphorylation. Eur. J. Cell Biol. 69(Suppl. 42):39.
-
- Ahern-Djamali SM, Comer AR, Bachmann C, Kastenmeier AS, Reddy SK, Beckerle MC, Walter U, Hoffmann FM. Mutations in DrosophilaEnabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol Biol Cell. 1998;9:2157–2171. - PMC - PubMed
-
- Aszodi A, Pfeifer A, Ahmad M, Glauner M, Zhou X-H, Ny L, Andersson K-E, Kehrel B, Offermans S, Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation but is dispensable for smooth muscle function. EMBO (Eur Mol Biol Organ) J. 1999;18:37–48. - PMC - PubMed
-
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitroand in intact human platelets. J Biol Chem. 1994;269:14509–14517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

