Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails
- PMID: 10087269
- PMCID: PMC2150570
- DOI: 10.1083/jcb.144.6.1271
Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails
Abstract
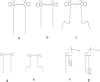
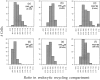
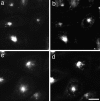
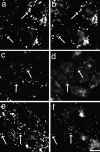
To understand the mechanisms for endocytic sorting of lipids, we investigated the trafficking of three lipid-mimetic dialkylindocarbocyanine (DiI) derivatives, DiIC16(3) (1,1'-dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate), DiIC12(3) (1,1'- didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate), and FAST DiI (1,1'-dilinoleyl-3,3,3', 3'-tetramethylindocarbocyanine perchlorate), in CHO cells by quantitative fluorescence microscopy. All three DiIs have the same head group, but differ in their alkyl tail length or unsaturation; these differences are expected to affect their distribution in membrane domains of varying fluidity or curvature. All three DiIs initially enter sorting endosomes containing endocytosed transferrin. DiIC16(3), with two long 16-carbon saturated tails is then delivered to late endosomes, whereas FAST DiI, with two cis double bonds in each tail, and DiIC12(3), with saturated but shorter (12-carbon) tails, are mainly found in the endocytic recycling compartment. We also find that DiOC16(3) (3,3'- dihexadecyloxacarbocyanine perchlorate) and FAST DiO (3, 3'-dilinoleyloxacarbocyanine perchlorate) behave similarly to their DiI counterparts. Furthermore, whereas a phosphatidylcholine analogue with a BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) fluorophore attached at the end of a 5-carbon acyl chain is delivered efficiently to the endocytic recycling compartment, a significant fraction of another derivative with BODIPY attached to a 12-carbon acyl chain entered late endosomes. Our results thus suggest that endocytic organelles can sort membrane components efficiently based on their preference for association with domains of varying characteristics.
Figures
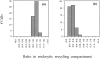






References
-
- Brown DA, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? . Biochem Biophys Res Commun. 1997;240:1–7. - PubMed
-
- Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987;26:39–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

