Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function
- PMID: 10087275
- PMCID: PMC2150590
- DOI: 10.1083/jcb.144.6.1349
Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function
Abstract
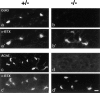
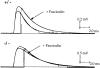
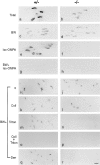
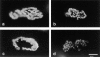
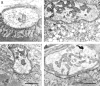
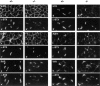
Acetylcholinesterase (AChE) occurs in both asymmetric forms, covalently associated with a collagenous subunit called Q (ColQ), and globular forms that may be either soluble or membrane associated. At the skeletal neuromuscular junction, asymmetric AChE is anchored to the basal lamina of the synaptic cleft, where it hydrolyzes acetylcholine to terminate synaptic transmission. AChE has also been hypothesized to play developmental roles in the nervous system, and ColQ is also expressed in some AChE-poor tissues. To seek roles of ColQ and AChE at synapses and elsewhere, we generated ColQ-deficient mutant mice. ColQ-/- mice completely lacked asymmetric AChE in skeletal and cardiac muscles and brain; they also lacked asymmetric forms of the AChE homologue, butyrylcholinesterase. Thus, products of the ColQ gene are required for assembly of all detectable asymmetric AChE and butyrylcholinesterase. Surprisingly, globular AChE tetramers were also absent from neonatal ColQ-/- muscles, suggesting a role for the ColQ gene in assembly or stabilization of AChE forms that do not themselves contain a collagenous subunit. Histochemical, immunohistochemical, toxicological, and electrophysiological assays all indicated absence of AChE at ColQ-/- neuromuscular junctions. Nonetheless, neuromuscular function was initially robust, demonstrating that AChE and ColQ do not play obligatory roles in early phases of synaptogenesis. Moreover, because acute inhibition of synaptic AChE is fatal to normal animals, there must be compensatory mechanisms in the mutant that allow the synapse to function in the chronic absence of AChE. One structural mechanism appears to be a partial ensheathment of nerve terminals by Schwann cells. Compensation was incomplete, however, as animals lacking ColQ and synaptic AChE failed to thrive and most died before they reached maturity.
Figures




References
-
- Andres C, Beeri R, Friedman A, Lev-Lehman E, Henis S, Timberg R, Shani M, Soreq H. Acetylcholinesterase-transgenic mice display embryonic modulations in spinal cord choline acetyltransferase and neurexin I beta gene expression followed by late-onset neuromotor deterioration. Proc Natl Acad Sci USA. 1997;94:8173–8178. - PMC - PubMed
-
- Bon S, Massoulié J. Quaternary association of acetylcholinesterase. I. Oligomeric associations of T subunits with and without the amino-terminal domain of the collagen tail. J Biol Chem. 1997;272:3007–3015. - PubMed
-
- Bon S, Coussen F, Massoulié J. Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J Biol Chem. 1997;272:3016–3021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

