Quantal size is correlated with receptor cluster area at glycinergic synapses in the rat brainstem
- PMID: 10087348
- PMCID: PMC2269264
- DOI: 10.1111/j.1469-7793.1999.0505v.x
Quantal size is correlated with receptor cluster area at glycinergic synapses in the rat brainstem
Abstract
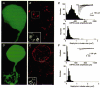
1. Whole-cell patch electrode recordings of glycinergic miniature inhibitory postsynaptic currents (mIPSCs) were obtained in neurons of the rat anteroventral cochlear nucleus (AVCN). Mean mIPSC peak amplitude was found to vary considerably between AVCN neurons (range, -19.1 to -317.9 pA; mean +/- s.d., -159.1 +/- 100.7 pA; 14 cells). 2. Immunolabelling of glycinergic receptor clusters in AVCN neurons was performed using antibodies against the glycine receptor clustering protein gephyrin. Measurements of the area of gephyrin immunoreactive clusters were obtained using confocal fluorescence microscopy. These measurements showed a large variability in cluster area, not only in the same cell (mean coefficient of variation, c.v., 0.66 +/- 0.18; 16 cells), but also in mean cluster area between cells (range, 0.21-0.84 microm2; 16 cells). 3. A possible relationship between mIPSC amplitude and receptor cluster area was investigated in a further series of experiments, in which mIPSCs recordings and immunolabelling of glycine receptor clusters were obtained for the same cells. In these experiments, AVCN neurons were identified using intracellular labelling with neurobiotin. Successful results using a combination of whole-cell recordings, neurobiotin identification and immunolabelling were obtained for a total of 10 AVCN neurons. Analysis of the results revealed a positive, statistically significant correlation between mean receptor cluster size and mean mIPSC amplitude (P < 0.05, 10 cells, Spearman's correlation test). 4. These results provide direct experimental evidence supporting a hypothesis of central glycinergic transmission in which synaptic strength may be regulated by changes in the size of the postsynaptic receptor region.
Figures



References
-
- Calverley RK, Jones DG. Contributions of dendritic spines and perforated synapses to synaptic plasticity. Brain Research Reviews. 1990;15:215–249. - PubMed
-
- Clements JD. Transmitter time course in the synaptic cleft: its role in central synaptic function? Trends in Neurosciences. 1996;19:163–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

