Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo
- PMID: 10087352
- PMCID: PMC2269258
- DOI: 10.1111/j.1469-7793.1999.0549v.x
Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo
Abstract
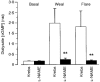
1. Nitric oxide (NO) concentrations were measured in dialysate from healthy human skin, in vivo, both at rest and during the inflammatory response to intradermal histamine or bradykinin. Changes in dialysate NO concentration, measured by electrochemical detection, were related to changes in dermal vascular perfusion, measured using scanning laser Doppler imaging. 2. Basal NO concentration in dermal microdialysate was 0.60 +/- 0.14 microM (mean +/- s.e.m.). Following the intradermal injection of histamine, a transient, time-dependent increase in NO concentration was measured in areas of skin incorporating the weal and in others incorporating the flare. The increase in NO concentration was associated with an increase in dialysate cGMP concentration in both the weal and flare areas. 3. Addition of N G-nitro-l-arginine-methyl ester (L-NAME, 5 mM) to the probe perfusate resulted in an inhibition of the histamine-induced increase in NO and cGMP. Moreover, the reduction in dialysate NO concentration was associated with a reduction in dermal vascular flux, both under basal conditions and within the weal and flare response. 4. These results demonstrate, by the use of microdialysis, that vasoactive mediators can be measured in healthy human skin in vivo. They provide direct evidence that endogenous concentration of NO increases during the inflammatory weal and flare response to histamine and that the increase in dermal NO concentration is associated with increases in cGMP concentration and dermal vascular perfusion, thus confirming a role for NO in vasoregulation in human skin.
Figures
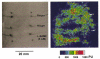
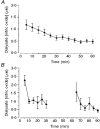
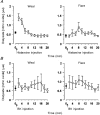
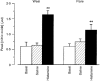
 ). Dialysate was collected from both the weal and flare areas of the histamine response. Maximum values in the weal occurred 2-4 min (P < 0·001) after provocation with histamine and that in the flare 6-8 min (P < 0·01). All columns represent the means +
). Dialysate was collected from both the weal and flare areas of the histamine response. Maximum values in the weal occurred 2-4 min (P < 0·001) after provocation with histamine and that in the flare 6-8 min (P < 0·01). All columns represent the means +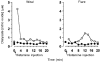
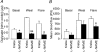
References
-
- Benrath J, Eschenfelder C, Zimmerman M, Gillardon F. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. European Journal of Pharmacology. 1995;293:87–96. - PubMed
-
- Bito L, Dawson H, Levin E, Murray M, Snider N. The concentrations of free amino acids and other electrolytes in cerebral spinal fluid, in vivo dialysate of brain, and blood plasma of the dog. Journal of Neurological Chemistry. 1966;13:1057–1067. - PubMed
-
- Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. Journal of Investigative Dermatology. 1998;110:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

