Contaminant bioavailability in soils, sediments, and aquatic environments
- PMID: 10097045
- PMCID: PMC34276
- DOI: 10.1073/pnas.96.7.3365
Contaminant bioavailability in soils, sediments, and aquatic environments
Abstract
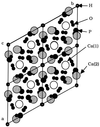
The aqueous concentrations of heavy metals in soils, sediments, and aquatic environments frequently are controlled by the dissolution and precipitation of discrete mineral phases. Contaminant uptake by organisms as well as contaminant transport in natural systems typically occurs through the solution phase. Thus, the thermodynamic solubility of contaminant-containing minerals in these environments can directly influence the chemical reactivity, transport, and ecotoxicity of their constituent ions. In many cases, Pb-contaminated soils and sediments contain the minerals anglesite (PbSO4), cerussite (PbCO3), and various lead oxides (e.g., litharge, PbO) as well as Pb2+ adsorbed to Fe and Mn (hydr)oxides. Whereas adsorbed Pb can be comparatively inert, the lead oxides, sulfates, and carbonates are all highly soluble in acidic to circumneutral environments, and soil Pb in these forms can pose a significant environmental risk. In contrast, the lead phosphates [e.g., pyromorphite, Pb5(PO4)3Cl] are much less soluble and geochemically stable over a wide pH range. Application of soluble or solid-phase phosphates (i.e., apatites) to contaminated soils and sediments induces the dissolution of the "native" Pb minerals, the desorption of Pb adsorbed by hydrous metal oxides, and the subsequent formation of pyromorphites in situ. This process results in decreases in the chemical lability and bioavailability of the Pb without its removal from the contaminated media. This and analogous approaches may be useful strategies for remediating contaminated soils and sediments.
Figures



References
-
- Krauskopf K B, Bird D K. Introduction to Geochemistry. New York: McGraw-Hill; 1995.
-
- Hayes K F, Traina S J. In: Soil Chemistry and Ecosystem Health. Huang P M, editor. Madison, WI: Soil Sci. Soc. Am.; 1998. pp. 45–84.
-
- Newman M C, McIntosh A W. Metal Ecotoxicology Concepts and Application. Chelsea, MI: Lewis; 1991.
-
- Adriano D C. Biogeochemistry of Trace Metal. Chelsea, MI: Lewis; 1992.
-
- Iskandar I K, Selim H M. Engineering Aspects of Metal-Waste Management. Chelsea, MI: Lewis; 1992.
LinkOut - more resources
Full Text Sources
Other Literature Sources

