Airborne minerals and related aerosol particles: effects on climate and the environment
- PMID: 10097046
- PMCID: PMC34277
- DOI: 10.1073/pnas.96.7.3372
Airborne minerals and related aerosol particles: effects on climate and the environment
Abstract
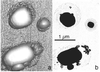
Aerosol particles are ubiquitous in the troposphere and exert an important influence on global climate and the environment. They affect climate through scattering, transmission, and absorption of radiation as well as by acting as nuclei for cloud formation. A significant fraction of the aerosol particle burden consists of minerals, and most of the remainder- whether natural or anthropogenic-consists of materials that can be studied by the same methods as are used for fine-grained minerals. Our emphasis is on the study and character of the individual particles. Sulfate particles are the main cooling agents among aerosols; we found that in the remote oceanic atmosphere a significant fraction is aggregated with soot, a material that can diminish the cooling effect of sulfate. Our results suggest oxidization of SO2 may have occurred on soot surfaces, implying that even in the remote marine troposphere soot provided nuclei for heterogeneous sulfate formation. Sea salt is the dominant aerosol species (by mass) above the oceans. In addition to being important light scatterers and contributors to cloud condensation nuclei, sea-salt particles also provide large surface areas for heterogeneous atmospheric reactions. Minerals comprise the dominant mass fraction of the atmospheric aerosol burden. As all geologists know, they are a highly heterogeneous mixture. However, among atmospheric scientists they are commonly treated as a fairly uniform group, and one whose interaction with radiation is widely assumed to be unpredictable. Given their abundances, large total surface areas, and reactivities, their role in influencing climate will require increased attention as climate models are refined.
Figures





References
-
- Farquhar G D. Science. 1997;278:1411.
-
- Mahlman J D. Science. 1997;278:1416–1417.
-
- Molina M J, Molina L T, Kolb C E. Annu Rev Phys Chem. 1996;47:327–367.
-
- Charlson R J, Heintzenberg J, editors. Aerosol Forcing of Climate. New York: Wiley; 1995.
-
- Houghton J T, Meiro Filho L G, Callander B A, Harris N, Kattenberg A, Maskell K, editors. Climate Change 1995: The Science of Climate Change, Contribution of Working Group I to the Second Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) Cambridge, U. K.: Cambridge Univ. Press; 1996.
LinkOut - more resources
Full Text Sources
Research Materials

