Designing conditions for in vitro formation of amyloid protofilaments and fibrils
- PMID: 10097081
- PMCID: PMC22338
- DOI: 10.1073/pnas.96.7.3590
Designing conditions for in vitro formation of amyloid protofilaments and fibrils
Abstract
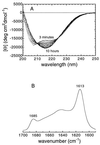
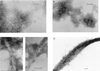
We have been able to convert a small alpha/beta protein, acylphosphatase, from its soluble and native form into insoluble amyloid fibrils of the type observed in a range of pathological conditions. This was achieved by allowing slow growth in a solution containing moderate concentrations of trifluoroethanol. When analyzed with electron microscopy, the protein aggregate present in the sample after long incubation times consisted of extended, unbranched filaments of 30-50 A in width that assemble subsequently into higher order structures. This fibrillar material possesses extensive beta-sheet structure as revealed by far-UV CD and IR spectroscopy. Furthermore, the fibrils exhibit Congo red birefringence, increased fluorescence with thioflavine T and cause a red-shift of the Congo red absorption spectrum. All of these characteristics are typical of amyloid fibrils. The results indicate that formation of amyloid occurs when the native fold of a protein is destabilized under conditions in which noncovalent interactions, and in particular hydrogen bonding, within the polypeptide chain remain favorable. We suggest that amyloid formation is not restricted to a small number of protein sequences but is a property common to many, if not all, natural polypeptide chains under appropriate conditions.
Figures

Comment in
-
Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease.Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3342-4. doi: 10.1073/pnas.96.7.3342. Proc Natl Acad Sci U S A. 1999. PMID: 10097040 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

