Expression of Batis maritima methyl chloride transferase in Escherichia coli
- PMID: 10097085
- PMCID: PMC22342
- DOI: 10.1073/pnas.96.7.3611
Expression of Batis maritima methyl chloride transferase in Escherichia coli
Abstract
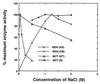
Methyl chloride transferase, a novel enzyme found in several fungi, marine algae, and halophytic plants, is a biological catalyst responsible for the production of atmospheric methyl chloride. A previous paper reports the purification of this methylase from Batis maritima and the isolation of a cDNA clone of the gene for this enzyme. In this paper, we describe the isolation of a genomic clone of the methylase gene and the expression of recombinant methyl chloride transferase in Escherichia coli and compare the kinetic behavior of the wild-type and recombinant enzyme. The recombinant enzyme is active and promotes the production of methyl chloride by E. coli under in vivo conditions. The kinetic data indicate that the recombinant and wild-type enzymes have similar halide (Cl-, Br-, and I-)-binding capacities. Both the recombinant and wild-type enzymes were found to function well in high NaCl concentrations. This high salt tolerance resembles the activity of halobacterial enzymes rather than halophytic plant enzymes. These findings support the hypothesis that this enzyme functions in the control and regulation of the internal concentration of chloride ions in halophytic plant cells.
Figures



References
-
- Rasmussen R A, Rasmussen L E, Kahlil M A K, Dalluge R W. J Geophys Res. 1980;85:7350–7353.
-
- Harper D B. Nature (London) 1985;315:55–57.
-
- Wuuosmaa A, Hager L P. Science. 1990;249:160–162. - PubMed
-
- Wuuosmaa A. Ph.D. thesis. Urbana, IL: Univ. of Illinois; 1994.
-
- Gschwend P W, MacFarlane J K, Newman K A. Science. 1985;227:1033–1035. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

