Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
- PMID: 10097096
- PMCID: PMC22353
- DOI: 10.1073/pnas.96.7.3676
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
Abstract
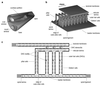
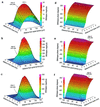
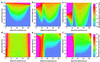
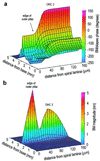
Normal mammalian hearing is refined by amplification of the motion of the cochlear partition. This partition, comprising the organ of Corti sandwiched between the basilar and tectorial membranes, contains the outer hair cells that are thought to drive this amplification process. Force generation by outer hair cells has been studied extensively in vitro and in situ, but, to understand cochlear amplification fully, it is necessary to characterize the role played by each of the components of the cochlear partition in vivo. Observations of cochlear partition motion in vivo are severely restricted by its inaccessibility and sensitivity to surgical trauma, so, for the present study, a computer model has been used to simulate the operation of the cochlea under different experimental conditions. In this model, which uniquely retains much of the three-dimensional complexity of the real cochlea, the motions of the basilar and tectorial membranes are fundamentally different during in situ- and in vivo-like conditions. Furthermore, enhanced outer hair cell force generation in vitro leads paradoxically to a decrease in the gain of the cochlear amplifier during sound stimulation to the model in vivo. These results suggest that it is not possible to extrapolate directly from experimental observations made in vitro and in situ to the normal operation of the intact organ in vivo.
Figures

References
-
- Cooper N P, Rhode W S. J Neurophysiol. 1997;78:261–270. - PubMed
-
- Khanna S M, Leonard D G B. Science. 1982;215:305–306. - PubMed
-
- Nuttall A L, Dolan D F, Avinash G. Hear Res. 1991;51:203–214. - PubMed
-
- Rhode W S. J Acoust Soc Am. 1971;49:1218–1231. - PubMed
-
- Robles L, Ruggero M, Rich N. J Acoust Soc Am. 1986;80:1364–1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

