GADD45 induction of a G2/M cell cycle checkpoint
- PMID: 10097101
- PMCID: PMC22358
- DOI: 10.1073/pnas.96.7.3706
GADD45 induction of a G2/M cell cycle checkpoint
Abstract
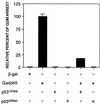
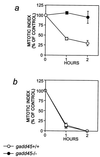
G1/S and G2/M cell cycle checkpoints maintain genomic stability in eukaryotes in response to genotoxic stress. We report here both genetic and functional evidence of a Gadd45-mediated G2/M checkpoint in human and murine cells. Increased expression of Gadd45 via microinjection of an expression vector into primary human fibroblasts arrests the cells at the G2/M boundary with a phenotype of MPM2 immunopositivity, 4n DNA content and, in 15% of the cells, centrosome separation. The Gadd45-mediated G2/M arrest depends on wild-type p53, because no arrest was observed either in p53-null Li-Fraumeni fibroblasts or in normal fibroblasts coexpressed with p53 mutants. Increased expression of cyclin B1 and Cdc25C inhibited the Gadd45-mediated G2/M arrest in human fibroblasts, indicating that the mechanism of Gadd45-mediated G2/M checkpoint is at least in part through modulation of the activity of the G2-specific kinase, cyclin B1/p34(cdc2). Genetic and physiological evidence of a Gadd45-mediated G2/M checkpoint was obtained by using GADD45-deficient human or murine cells. Human cells with endogenous Gadd45 expression reduced by antisense GADD45 expression have an impaired G2/M checkpoint after exposure to either ultraviolet radiation or methyl methanesulfonate but are still able to undergo G2 arrest after ionizing radiation. Lymphocytes from gadd45-knockout mice (gadd45 -/-) also retained a G2/M checkpoint initiated by ionizing radiation and failed to arrest at G2/M after exposure to ultraviolet radiation. Therefore, the mammalian genome is protected by a multiplicity of G2/M checkpoints in response to specific types of DNA damage.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

