Import of DNA into mammalian nuclei by proteins originating from a plant pathogenic bacterium
- PMID: 10097105
- PMCID: PMC22362
- DOI: 10.1073/pnas.96.7.3729
Import of DNA into mammalian nuclei by proteins originating from a plant pathogenic bacterium
Abstract
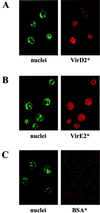
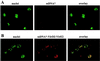
Import of DNA into mammalian nuclei is generally inefficient. Therefore, one of the current challenges in human gene therapy is the development of efficient DNA delivery systems. Here we tested whether bacterial proteins could be used to target DNA to mammalian cells. Agrobacterium tumefaciens, a plant pathogen, efficiently transfers DNA as a nucleoprotein complex to plant cells. Agrobacterium-mediated T-DNA transfer to plant cells is the only known example for interkingdom DNA transfer and is widely used for plant transformation. Agrobacterium virulence proteins VirD2 and VirE2 perform important functions in this process. We reconstituted complexes consisting of the bacterial virulence proteins VirD2, VirE2, and single-stranded DNA (ssDNA) in vitro. These complexes were tested for import into HeLa cell nuclei. Import of ssDNA required both VirD2 and VirE2 proteins. A VirD2 mutant lacking its C-terminal nuclear localization signal was deficient in import of the ssDNA-protein complexes into nuclei. Import of VirD2-ssDNA-VirE2 complexes was fast and efficient, and was shown to depended on importin alpha, Ran, and an energy source. We report here that the bacterium-derived and plant-adapted protein-DNA complex, made in vitro, can be efficiently imported into mammalian nuclei following the classical importin-dependent nuclear import pathway. This demonstrates the potential of our approach to enhance gene transfer to animal cells.
Figures

Similar articles
-
Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins.Plant Cell. 2001 Feb;13(2):369-83. doi: 10.1105/tpc.13.2.369. Plant Cell. 2001. PMID: 11226191 Free PMC article.
-
Agrobacterium tumefaciens and A. rhizogenes use different proteins to transport bacterial DNA into the plant cell nucleus.Microb Biotechnol. 2009 Jul;2(4):416-27. doi: 10.1111/j.1751-7915.2009.00104.x. Epub 2009 Apr 6. Microb Biotechnol. 2009. PMID: 21255274 Free PMC article. Review.
-
Agrobacterium-delivered VirE2 interacts with host nucleoporin CG1 to facilitate the nuclear import of VirE2-coated T complex.Proc Natl Acad Sci U S A. 2020 Oct 20;117(42):26389-26397. doi: 10.1073/pnas.2009645117. Epub 2020 Oct 5. Proc Natl Acad Sci U S A. 2020. PMID: 33020260 Free PMC article.
-
Functional domains of Agrobacterium tumefaciens single-stranded DNA-binding protein VirE2.J Bacteriol. 1997 Feb;179(4):1165-73. doi: 10.1128/jb.179.4.1165-1173.1997. J Bacteriol. 1997. PMID: 9023198 Free PMC article.
-
The VirE2 protein of Agrobacterium tumefaciens: the Yin and Yang of T-DNA transfer.FEMS Microbiol Lett. 2003 Jun 6;223(1):1-6. doi: 10.1016/S0378-1097(03)00246-5. FEMS Microbiol Lett. 2003. PMID: 12798992 Review.
Cited by
-
Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins.Plant Cell. 2001 Feb;13(2):369-83. doi: 10.1105/tpc.13.2.369. Plant Cell. 2001. PMID: 11226191 Free PMC article.
-
The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation.Int J Dev Biol. 2013;57(6-8):467-81. doi: 10.1387/ijdb.130199bl. Int J Dev Biol. 2013. PMID: 24166430 Free PMC article. Review.
-
Agrobacterium tumefaciens and A. rhizogenes use different proteins to transport bacterial DNA into the plant cell nucleus.Microb Biotechnol. 2009 Jul;2(4):416-27. doi: 10.1111/j.1751-7915.2009.00104.x. Epub 2009 Apr 6. Microb Biotechnol. 2009. PMID: 21255274 Free PMC article. Review.
-
Pathways of DNA Transfer to Plants from Agrobacterium tumefaciens and Related Bacterial Species.Annu Rev Phytopathol. 2019 Aug 25;57:231-251. doi: 10.1146/annurev-phyto-082718-100101. Epub 2019 Jun 21. Annu Rev Phytopathol. 2019. PMID: 31226020 Free PMC article.
-
Kinetics and mechanism of DNA uptake into the cell nucleus.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7247-52. doi: 10.1073/pnas.121067698. Epub 2001 Jun 5. Proc Natl Acad Sci U S A. 2001. PMID: 11390964 Free PMC article.
References
-
- Lartey R, Citovsky V. Genet Eng (NY) 1997;19:201–214. - PubMed
-
- Zupan J, Zambryski P. Crit Rev Plant Sci. 1997;16:279–295.
-
- Rossi L, Tinland B, Hohn B. In: The Rhiziobiaceae. Spaink H, Hooykaas P, Kondorosi A, editors. Boston: Kluwer; 1998. pp. 302–330.
-
- Tinland B, Hohn B. Genet Eng (NY) 1995;17:209–229. - PubMed
-
- Tinland B. Trends Plant Sci. 1996;1:178–184.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

