Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1
- PMID: 10097128
- PMCID: PMC22385
- DOI: 10.1073/pnas.96.7.3859
Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1
Abstract
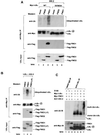
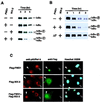
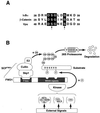
Activation of the transcription factor nuclear factor kappa B (NF-kappaB) is controlled by proteolysis of its inhibitory subunit (IkappaB) via the ubiquitin-proteasome pathway. Signal-induced phosphorylation of IkappaBalpha by a large multisubunit complex containing IkappaB kinases is a prerequisite for ubiquitination. Here, we show that FWD1 (a mouse homologue of Slimb/betaTrCP), a member of the F-box/WD40-repeat proteins, is associated specifically with IkappaBalpha only when IkappaBalpha is phosphorylated. The introduction of FWD1 into cells significantly promotes ubiquitination and degradation of IkappaBalpha in concert with IkappaB kinases, resulting in nuclear translocation of NF-kappaB. In addition, FWD1 strikingly evoked the ubiquitination of IkappaBalpha in the in vitro system. In contrast, a dominant-negative form of FWD1 inhibits the ubiquitination, leading to stabilization of IkappaBalpha. These results suggest that the substrate-specific degradation of IkappaBalpha is mediated by a Skp1/Cull 1/F-box protein (SCF) FWD1 ubiquitin-ligase complex and that FWD1 serves as an intracellular receptor for phosphorylated IkappaBalpha. Skp1/Cullin/F-box protein FWD1 might play a critical role in transcriptional regulation of NF-kappaB through control of IkappaB protein stability.
Figures

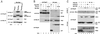
Similar articles
-
Molecular dissection of the interactions among IkappaBalpha, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IkappaBalpha.J Biol Chem. 1999 Oct 15;274(42):29641-7. doi: 10.1074/jbc.274.42.29641. J Biol Chem. 1999. PMID: 10514433
-
Common pathway for the ubiquitination of IkappaBalpha, IkappaBbeta, and IkappaBepsilon mediated by the F-box protein FWD1.J Biol Chem. 1999 Oct 1;274(40):28169-74. doi: 10.1074/jbc.274.40.28169. J Biol Chem. 1999. PMID: 10497169
-
IkappaBalpha ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, betaTrCP1 and betaTrCP2.Biochem Biophys Res Commun. 1999 Mar 5;256(1):127-32. doi: 10.1006/bbrc.1999.0289. Biochem Biophys Res Commun. 1999. PMID: 10066435
-
Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway.Biochimie. 2001 Mar-Apr;83(3-4):351-6. doi: 10.1016/s0300-9084(01)01237-8. Biochimie. 2001. PMID: 11295496 Review.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
TSPAN15 interacts with BTRC to promote oesophageal squamous cell carcinoma metastasis via activating NF-κB signaling.Nat Commun. 2018 Apr 12;9(1):1423. doi: 10.1038/s41467-018-03716-9. Nat Commun. 2018. PMID: 29650964 Free PMC article.
-
Rapid temporal dynamics of transcription, protein synthesis, and secretion during macrophage activation.Mol Cell Proteomics. 2014 Mar;13(3):792-810. doi: 10.1074/mcp.M113.030916. Epub 2014 Jan 6. Mol Cell Proteomics. 2014. PMID: 24396086 Free PMC article.
-
A dominant-negative F-box deleted mutant of E3 ubiquitin ligase, β-TrCP1/FWD1, markedly reduces myeloma cell growth and survival in mice.Oncotarget. 2015 Aug 28;6(25):21589-602. doi: 10.18632/oncotarget.4120. Oncotarget. 2015. PMID: 26009993 Free PMC article.
-
TRIM22 activates NF-κB signaling in glioblastoma by accelerating the degradation of IκBα.Cell Death Differ. 2021 Jan;28(1):367-381. doi: 10.1038/s41418-020-00606-w. Epub 2020 Aug 19. Cell Death Differ. 2021. PMID: 32814880 Free PMC article.
-
Early embryonic death in mice lacking the beta-catenin-binding protein Duplin.Mol Cell Biol. 2004 Oct;24(19):8386-94. doi: 10.1128/MCB.24.19.8386-8394.2004. Mol Cell Biol. 2004. PMID: 15367660 Free PMC article.
References
-
- Hershko A, Ciechanover A. Annu Rev Biochem. 1992;61:761–807. - PubMed
-
- Weissman A M. Immunol Today. 1997;18:189–198. - PubMed
-
- Elledge S J, Harper J W. Biochim Biophys Acta. 1998;1377:M61–M70. - PubMed
-
- Krek W. Curr Opin Genet Dev. 1998;8:36–42. - PubMed
-
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper J W, Elledge S J. Cell. 1996;86:263–274. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

