Regulation of T cell receptor (TCR) beta gene expression by CD3 complex signaling in immature thymocytes: implications for TCRbeta allelic exclusion
- PMID: 10097132
- PMCID: PMC22389
- DOI: 10.1073/pnas.96.7.3882
Regulation of T cell receptor (TCR) beta gene expression by CD3 complex signaling in immature thymocytes: implications for TCRbeta allelic exclusion
Abstract
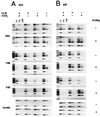
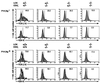
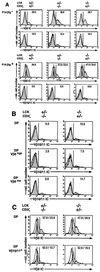
During alphabeta thymocyte development, clonotype-independent CD3 complexes are expressed at the cell surface before the pre-T cell receptor (TCR). Signaling through clonotype-independent CD3 complexes is required for expression of rearranged TCRbeta genes. On expression of a TCRbeta polypeptide chain, the pre-TCR is assembled, and TCRbeta locus allelic exclusion is established. We investigated the putative contribution of clonotype-independent CD3 complex signaling to TCRbeta locus allelic exclusion in mice single-deficient or double-deficient for CD3zeta/eta and/or p56(lck). These mice display defects in the expression of endogenous TCRbeta genes in immature thymocytes, proportional to the severity of CD3 complex malfunction. Exclusion of endogenous TCRbeta VDJ (variable, diversity, joining) rearrangements by a functional TCRbeta transgene was severely compromised in the single-deficient and double-deficient mutant mice. In contrast to wild-type mice, most of the CD25(+) double-negative (DN) thymocytes of the mutant mice failed to express the TCRbeta transgene, suggesting defective expression of the TCRbeta transgene similar to endogenous TCRbeta genes. In the mutant mice, a proportion of CD25(+) DN thymocytes that failed to express the transgene expressed endogenous TCRbeta polypeptide chains. Many double-positive cells of the mutant mice coexpressed endogenous and transgenic TCRbeta chains or more than one endogenous TCRbeta chain. The data suggest that signaling through clonotype-independent CD3 complexes may contribute to allelic exclusion of the TCRbeta locus by inducing the expression of rearranged TCRbeta genes in CD25(+) DN thymocytes.
Figures

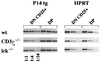
Similar articles
-
Requirement of CD3 complex-associated signaling functions for expression of rearranged T cell receptor beta VDJ genes in early thymic development.J Exp Med. 1998 Nov 2;188(9):1669-78. doi: 10.1084/jem.188.9.1669. J Exp Med. 1998. PMID: 9802979 Free PMC article.
-
Reduced generation but efficient TCR beta-chain selection of CD4+8+ double-positive thymocytes in mice with compromised CD3 complex signaling.J Immunol. 1999 Mar 1;162(5):2741-7. J Immunol. 1999. PMID: 10072519
-
Expression and selection of productively rearranged TCR beta VDJ genes are sequentially regulated by CD3 signaling in the development of NK1.1(+) alpha beta T cells.Int Immunol. 2001 Aug;13(8):1031-42. doi: 10.1093/intimm/13.8.1031. Int Immunol. 2001. PMID: 11470773
-
Allelic exclusion at the TCRbeta locus.Curr Opin Immunol. 2002 Apr;14(2):230-4. doi: 10.1016/s0952-7915(02)00326-6. Curr Opin Immunol. 2002. PMID: 11869897 Review.
-
Regulation of thymocyte differentiation: pre-TCR signals and beta-selection.Semin Immunol. 2002 Oct;14(5):311-23. doi: 10.1016/s1044-5323(02)00064-7. Semin Immunol. 2002. PMID: 12220932 Review.
Cited by
-
Early T cell receptor beta gene expression is regulated by the pre-T cell receptor-CD3 complex.J Exp Med. 1999 Jul 5;190(1):141-4. doi: 10.1084/jem.190.1.141. J Exp Med. 1999. PMID: 10429678 Free PMC article.
-
EMBO WORKSHOP REPORT: lymphocyte antigen receptor and coreceptor signaling Siena, Italy, November 6-10, 1999.EMBO J. 2000 Sep 15;19(18):4857-65. doi: 10.1093/emboj/19.18.4857. EMBO J. 2000. PMID: 10990449 Free PMC article. No abstract available.
-
TCR beta feedback signals inhibit the coupling of recombinationally accessible V beta 14 segments with DJ beta complexes.J Immunol. 2010 Feb 1;184(3):1369-78. doi: 10.4049/jimmunol.0900723. Epub 2009 Dec 30. J Immunol. 2010. PMID: 20042591 Free PMC article.
References
-
- Levelt C N, Eichmann K. Immunity. 1995;3:667–672. - PubMed
-
- Fehling H J, von Boehmer H. Curr Opin Immunol. 1997;9:263–275. - PubMed
-
- Kisielow P, von Boehmer H. Adv Immunol. 1995;58:87–209. - PubMed
-
- Robey E, Fowlkes B J. Annu Rev Immunol. 1994;12:675–705. - PubMed
-
- Zunicka-Pflücker J L, Lenardo M J. Curr Opin Immunol. 1996;8:215–224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

