Sustained correction of bleeding disorder in hemophilia B mice by gene therapy
- PMID: 10097136
- PMCID: PMC22393
- DOI: 10.1073/pnas.96.7.3906
Sustained correction of bleeding disorder in hemophilia B mice by gene therapy
Abstract
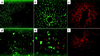
Mice generated by disrupting the clotting factor IX gene exhibit severe bleeding disorder and closely resemble the phenotype seen in hemophilia B patients. Here we demonstrate that a single intraportal injection of a recombinant adeno-associated virus (AAV) vector encoding canine factor IX cDNA under the control of a liver-specific enhancer/promoter leads to a long-term and complete correction of the bleeding disorder. High level expression of up to 15-20 microgram/ml of canine factor IX was detected in the plasma of mice injected with 5.6 x 10(11) particles of an AAV vector for >5 months. The activated partial thromboplastin time of the treated mice was fully corrected to higher than normal levels. Liver-specific expression of canine factor IX was confirmed by immunofluorescence staining, and secreted factor IX protein was identified in the mouse plasma by Western blotting. All treated mice survived the tail clip test without difficulty. Thus, a single intraportal injection of a recombinant adeno-associated virus vector expressing factor IX successfully cured the bleeding disorder of hemophilia B mice, proving the feasibility of using AAV-based vectors for liver-targeted gene therapy of genetic diseases.
Figures

References
-
- Lin H F, Maeda N, Smithies O, Straight D L, Stafford D W. Blood. 1997;90:3962–3966. - PubMed
-
- Kundu R K, Sangiorgi F, Wu L Y, Kurachi K, Anderson W F, Maxson R, Gordon E M. Blood. 1998;92:168–174. - PubMed
-
- Kung S H, Hagstrom J N, Cass D, Tai S J, Lin H F, Stafford D W, High K A. Blood. 1998;91:784–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

