Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome
- PMID: 10097145
- PMCID: PMC22402
- DOI: 10.1073/pnas.96.7.3957
Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome
Abstract
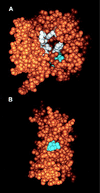
The dose-limiting toxicity of interleukin-2 (IL-2) and immunotoxin (IT) therapy in humans is vascular leak syndrome (VLS). VLS has a complex etiology involving damage to vascular endothelial cells (ECs), extravasation of fluids and proteins, interstitial edema, and organ failure. IL-2 and ITs prepared with the catalytic A chain of the plant toxin, ricin (RTA), and other toxins, damage human ECs in vitro and in vivo. Damage to ECs may initiate VLS; if this damage could be avoided without losing the efficacy of ITs or IL-2, larger doses could be administered. In this paper, we provide evidence that a three amino acid sequence motif, (x)D(y), in toxins and IL-2 damages ECs. Thus, when peptides from RTA or IL-2 containing this sequence motif are coupled to mouse IgG, they bind to and damage ECs both in vitro and, in the case of RTA, in vivo. In contrast, the same peptides with a deleted or mutated sequence do not. Furthermore, the peptide from RTA attached to mouse IgG can block the binding of intact RTA to ECs in vitro and vice versa. In addition, RTA, a fragment of Pseudomonas exotoxin A (PE38-lys), and fibronectin also block the binding of the mouse IgG-RTA peptide to ECs, suggesting that an (x)D(y) motif is exposed on all three molecules. Our results suggest that deletions or mutations in this sequence or the use of nondamaging blocking peptides may increase the therapeutic index of both IL-2, as well as ITs prepared with a variety of plant or bacterial toxins.
Figures

References
-
- Vitetta E S, Thorpe P E, Uhr J W. Immunol Today. 1993;14:252–259. - PubMed
-
- Sausville E A, Vitetta E S. In: Monoclonal Antibody-Based Therapy of Cancer. Grossbard M L, editor. Vol. 4. Basel: Marcel Dekker; 1997. pp. 81–89.
-
- Baluna R, Vitetta E S. Immunopharmacology. 1996;37:117–132. - PubMed
-
- Engert A, Sausville E A, Vitetta E S. In: Clinical Applications of Immunotoxins. Frankel A E, editor. Vol. 2. Berlin: Springer; 1997. pp. 13–33.
-
- Dutcher J P, Gaynor E R, Boldt D H, Doroshow J H, Bar M H, Sznol M, Mier J, Sparano J, Fisher R I, Weiss G, et al. J Clin Oncol. 1991;9:641–648. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

