Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen
- PMID: 10097148
- PMCID: PMC22405
- DOI: 10.1073/pnas.96.7.3975
Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen
Abstract
Angiotensin produced systemically or locally in tissues such as the brain plays an important role in the regulation of blood pressure and in the development of hypertension. We have established transgenic rats [TGR(ASrAOGEN)] expressing an antisense RNA against angiotensinogen mRNA specifically in the brain. In these animals, the brain angiotensinogen level is reduced by more than 90% and the drinking response to intracerebroventricular renin infusions is decreased markedly compared with control rats. Blood pressure of transgenic rats is lowered by 8 mmHg (1 mmHg = 133 Pa) compared with control rats. Crossbreeding of TGR(ASrAOGEN) with a hypertensive transgenic rat strain exhibiting elevated angiotensin II levels in tissues results in a marked attenuation of the hypertensive phenotype. Moreover, TGR(ASrAOGEN) exhibit a diabetes insipidus-like syndrome producing an increased amount of urine with decreased osmolarity. The observed reduction in plasma vasopressin by 35% may mediate these phenotypes of TGR(ASrAOGEN). This new animal model presenting long-term and tissue-specific down-regulation of angiotensinogen corroborates the functional significance of local angiotensin production in the brain for the central regulation of blood pressure and for the pathogenesis of hypertension.
Figures

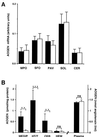

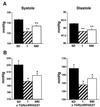
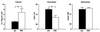
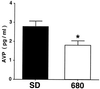
References
-
- Oparil S, Chen Y-F, Berecek K H, Calhoun D A, Wyss J M. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 713–740.
-
- Freis E D. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 2741–2751.
-
- Reid I A, Morris B J, Ganong W F. Annu Rev Physiol. 1978;40:377–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

