Hepatocyte gene therapy in a large animal: a neonatal bovine model of citrullinemia
- PMID: 10097149
- PMCID: PMC22406
- DOI: 10.1073/pnas.96.7.3981
Hepatocyte gene therapy in a large animal: a neonatal bovine model of citrullinemia
Abstract
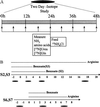
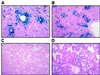
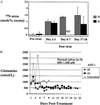
The development of gene-replacement therapy for inborn errors of metabolism has been hindered by the limited number of suitable large-animal models of these diseases and by inadequate methods of assessing the efficacy of treatment. Such methods should provide sensitive detection of expression in vivo and should be unaffected by concurrent pharmacologic and dietary regimens. We present the results of studies in a neonatal bovine model of citrullinemia, an inborn error of urea-cycle metabolism characterized by deficiency of argininosuccinate synthetase and consequent life-threatening hyperammonemia. Measurements of the flux of nitrogen from orally administered 15NH4 to [15N]urea were used to determine urea-cycle activity in vivo. In control animals, these isotopic measurements proved to be unaffected by pharmacologic treatments. Systemic administration of a first-generation E1-deleted adenoviral vector expressing human argininosuccinate synthetase resulted in transduction of hepatocytes and partial correction of the enzyme defect. The isotopic method showed significant restoration of urea synthesis. Moreover, the calves showed clinical improvement and normalization of plasma glutamine levels after treatment. The results show the clinical efficacy of treating a large-animal model of an inborn error of hepatocyte metabolism in conjunction with a method for sensitively measuring correction in vivo. These studies will be applicable to human trials of the treatment of this disorder and other related urea-cycle disorders.
Figures

References
-
- Brusilow S W, Horwich A L. In: The Molecular and Metabolic Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1187–1232.
-
- Batshaw M L, Brusilow S, Waber L, Blom W, Brubakk A M, Burton B K, Cann H M, Kerr D, Mamunes P, Matalon R, et al. N Engl J Med. 1982;306:1387–1392. - PubMed
-
- Brusilow S W, Danney M, Waber L J, Batshaw M, Burton B, Levitsky L, Roth K, McKeethren C, Ward J. N Engl J Med. 1984;310:1630–1634. - PubMed
-
- Msall M, Batshaw M L, Suss R, Brusilow S W, Mellits E D. N Engl J Med. 1984;310:1500–1505. - PubMed
-
- Bramson J L, Graham F L, Gauldie J. Curr Opin Biotechnol. 1995;6:590–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

