Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication
- PMID: 10097150
- PMCID: PMC22407
- DOI: 10.1073/pnas.96.7.3987
Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication
Abstract
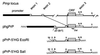
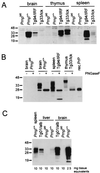
The cellular form of the Prion protein (PrPC) is necessary for prion replication in mice. To determine whether it is also sufficient, we expressed PrP under the control of various cell- or tissue-specific regulatory elements in PrP knockout mice. The interferon regulatory factor-1 promoter/Emu enhancer led to high PrP levels in the spleen and low PrP levels in the brain. Following i.p. scrapie inoculation, high prion titers were found in the spleen but not in the brain at 2 weeks and 6 months, showing that the lymphoreticular system by itself is competent to replicate prions. PrP expression directed by the Lck promoter resulted in high PrP levels on T lymphocytes only but, surprisingly, did not allow prion replication in the thymus, spleen, or brain following i.p. inoculation. A third transgenic line, which expressed PrP in the liver under the control of the albumin promoter/enhancer-albeit at low levels-also failed to replicate prions. These results show that expression of PrP alone is not sufficient to sustain prion replication and suggest that additional components are needed.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

