A mutant of Mycobacterium smegmatis defective in the biosynthesis of mycolic acids accumulates meromycolates
- PMID: 10097154
- PMCID: PMC22411
- DOI: 10.1073/pnas.96.7.4011
A mutant of Mycobacterium smegmatis defective in the biosynthesis of mycolic acids accumulates meromycolates
Abstract
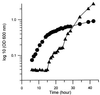
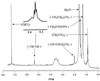
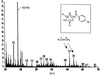
Mycolic acids are a major constituent of the mycobacterial cell wall, and they form an effective permeability barrier to protect mycobacteria from antimicrobial agents. Although the chemical structures of mycolic acids are well established, little is known on their biosynthesis. We have isolated a mycolate-deficient mutant strain of Mycobacterium smegmatis mc2-155 by chemical mutagenesis followed by screening for increased sensitivity to novobiocin. This mutant also was hypersensitive to other hydrophobic compounds such as crystal violet, rifampicin, and erythromycin. Entry of hydrophobic probes into mutant cells occurred much more rapidly than that into the wild-type cells. HPLC and TLC analysis of fatty acid composition after saponification showed that the mutant failed to synthesize full-length mycolic acids. Instead, it accumulated a series of long-chain fatty acids, which were not detected in the wild-type strain. Analysis by 1H NMR, electrospray and electron impact mass spectroscopy, and permanganate cleavage of double bonds showed that these compounds corresponded to the incomplete meromycolate chain of mycolic acids, except for the presence of a beta-hydroxyl group. This direct identification of meromycolates as precursors of mycolic acids provides a strong support for the previously proposed pathway for mycolic acid biosynthesis involving the separate synthesis of meromycolate chain and the alpha-branch of mycolic acids, followed by the joining of these two branches.
Figures




References
-
- Bloom B R, Murray C J L. Science. 1992;257:1055–1064. - PubMed
-
- Frieden T R, Sterling T, Pablos-Mendez A, Kilburn J O, Cauthen G M, Dooley S W. New Engl J Med. 1993;328:521–526. - PubMed
-
- Chan I S F, Neaton J D, Saravolatz L D, Crane L R, Osterberger J. AIDS. 1995;9:1145–1151. - PubMed
-
- Jarlier V, Nikaido H. FEMS Microbiol Lett. 1994;123:1–8. - PubMed
-
- Liu, J., Barry, C. E., III & Nikaido, H. in Mycobacteria: Molecular Biology and Virulence, eds. Ratledge, C. & Dale, J. W. (Blackwell Scientific, Oxford), in press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

