A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease
- PMID: 10097181
- PMCID: PMC22438
- DOI: 10.1073/pnas.96.7.4164
A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease
Abstract
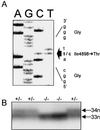
Central core disease is a rare, nonprogressive myopathy that is characterized by hypotonia and proximal muscle weakness. In a large Mexican kindred with an unusually severe and highly penetrant form of the disorder, DNA sequencing identified an I4898T mutation in the C-terminal transmembrane/luminal region of the RyR1 protein that constitutes the skeletal muscle ryanodine receptor. All previously reported RYR1 mutations are located either in the cytoplasmic N terminus or in a central cytoplasmic region of the 5,038-aa protein. The I4898T mutation was introduced into a rabbit RYR1 cDNA and expressed in HEK-293 cells. The response of the mutant RyR1 Ca2+ channel to the agonists halothane and caffeine in a Ca2+ photometry assay was completely abolished. Coexpression of normal and mutant RYR1 cDNAs in a 1:1 ratio, however, produced RyR1 channels with normal halothane and caffeine sensitivities, but maximal levels of Ca2+ release were reduced by 67%. [3H]Ryanodine binding indicated that the heterozygous channel is activated by Ca2+ concentrations 4-fold lower than normal. Single-cell analysis of cotransfected cells showed a significantly increased resting cytoplasmic Ca2+ level and a significantly reduced luminal Ca2+ level. These data are indicative of a leaky channel, possibly caused by a reduction in the Ca2+ concentration required for channel activation. Comparison with two other coexpressed mutant/normal channels suggests that the I4898T mutation produces one of the most abnormal RyR1 channels yet investigated, and this level of abnormality is reflected in the severe and penetrant phenotype of affected central core disease individuals.
Figures


Comment in
-
Luminal loop of the ryanodine receptor: a pore-forming segment?Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3345-7. doi: 10.1073/pnas.96.7.3345. Proc Natl Acad Sci U S A. 1999. PMID: 10097041 Free PMC article. Review. No abstract available.
References
-
- Shy G M, Magee K R. Brain. 1956;79:610–621. - PubMed
-
- Shuaib A, Paasuke R T, Brownell K W. Medicine. 1987;66:389–396. - PubMed
-
- Dubowitz V, Brooke M H. In: Major Problems in Neurology. Walton J N, editor. London: Saunders; 1973. pp. 92–119.
-
- Hayashi K, Miller R G, Brownell K W. Muscle Nerve. 1989;12:95–102. - PubMed
-
- Vita G, Migliorato A, Baradello A, Mazzeo A, Rodolico C, Falsaperla R, Messina C. J Neurol Sci. 1994;124:71–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

