2E4 (kaptin): a novel actin-associated protein from human blood platelets found in lamellipodia and the tips of the stereocilia of the inner ear
- PMID: 10099934
- PMCID: PMC3376092
- DOI: 10.1016/S0171-9335(99)80013-2
2E4 (kaptin): a novel actin-associated protein from human blood platelets found in lamellipodia and the tips of the stereocilia of the inner ear
Abstract
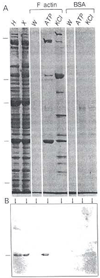
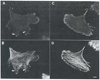
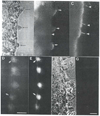
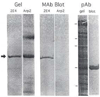
Platelet activation, crucial for hemostasis, requires actin polymerization, yet the molecular mechanisms by which localized actin polymerization is mediated are not clear. Here we report the characterization of a novel actin-binding protein, 2E4, originally isolated from human blood platelets and likely to be involved in the actin rearrangements occurring during activation. 2E4 binds to filamentous (F)-actin by F-actin affinity chromatography and is eluted from F-actin affinity columns and extracted from cells with ATP. Its presence at the leading edge of platelets spread on glass and in the lamellipodia of motile fibroblasts suggests a role in actin dynamics. Using localization to obtain clues about function, we stained the sensory epithelium of the embryonic inner ear to determine whether 2E4 is at the barbed end of actin filaments during their elongation. Indeed, 2E4 was present at the tips of the elongating stereocilium. 2E4 is novel by DNA sequence and has no identifiable structural motifs. Its unusual amino acid sequence, its ATP-sensitive actin association and its location at sites of actin polymerization in cells suggest 2E4 plays a unique role in the actin rearrangements that accompany platelet activation and stereocilia formation.
Figures





References
-
- Albanesi JP, Lynch TJ, Fujosaki H, Bowerts B, Korn ED. Purification and characterization of an ATP-sensitive actin gelation factor protein from Acanthamoeba castellani. J. Biol. Chem. 1987;262:3404–3408. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

