Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme
- PMID: 10102935
- PMCID: PMC2217165
- DOI: 10.1085/jgp.113.4.541
Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme
Abstract
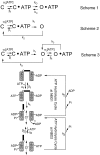
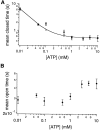
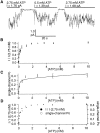
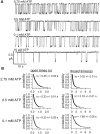
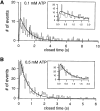
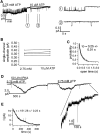
Gating of the cystic fibrosis transmembrane conductance regulator (CFTR) involves a coordinated action of ATP on two nucleotide binding domains (NBD1 and NBD2). Previous studies using nonhydrolyzable ATP analogues and NBD mutant CFTR have suggested that nucleotide hydrolysis at NBD1 is required for opening of the channel, while hydrolysis of nucleotides at NBD2 controls channel closing. We studied ATP-dependent gating of CFTR in excised inside-out patches from stably transfected NIH3T3 cells. Single channel kinetics of CFTR gating at different [ATP] were analyzed. The closed time constant (tauc) decreased with increasing [ATP] to a minimum value of approximately 0.43 s at [ATP] >1.00 mM. The open time constant (tauo) increased with increasing [ATP] with a minimal tauo of approximately 260 ms. Kinetic analysis of K1250A-CFTR, a mutant that abolishes ATP hydrolysis at NBD2, reveals the presence of two open states. A short open state with a time constant of approximately 250 ms is dominant at low ATP concentrations (10 microM) and a much longer open state with a time constant of approximately 3 min is present at millimolar ATP. These data suggest that nucleotide binding and hydrolysis at NBD1 is coupled to channel opening and that the channel can close without nucleotide interaction with NBD2. A quantitative cyclic gating scheme with microscopic irreversibility was constructed based on the kinetic parameters derived from single-channel analysis. The estimated values of the kinetic parameters suggest that NBD1 and NBD2 are neither functionally nor biochemically equivalent.
Figures


References
-
- Ames GF, Lecar F. ATP-dependent bacterial transporters and cystic fibrosis: analogy between channels and transporters. FASEB J. 1992;6:2660–2666. - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR channel. Cell. 1991a;67:775–784. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991b;253:202–205. - PubMed
-
- Baukrowitz T, Hwang T-C, Nairn AC, Gadsby DC. Coupling of CFTR Cl−channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. - PubMed
-
- Bear CE, Li C, Kartner N, Bridges RJ, Jensen TJ. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. - PubMed

