Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes
- PMID: 10102938
- PMCID: PMC2217167
- DOI: 10.1085/jgp.113.4.581
Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes
Abstract
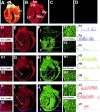
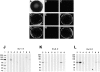
The biophysical characteristics and alpha subunits underlying calcium-independent transient outward potassium current (Ito) phenotypes expressed in ferret left ventricular epicardial (LV epi) and endocardial (LV endo) myocytes were analyzed using patch clamp, fluorescent in situ hybridization (FISH), and immunofluorescent (IF) techniques. Two distinct Ito phenotypes were measured (21-22 degrees C) in the majority of LV epi and LV endo myocytes studied. The two Ito phenotypes displayed marked differences in peak current densities, activation thresholds, inactivation characteristics, and recovery kinetics. Ito,epi recovered rapidly [taurec, -70 mV = 51 +/- 3 ms] with minimal cumulative inactivation, while Ito,endo recovered slowly [taurec, -70 mV = 3,002 +/- 447 ms] with marked cumulative inactivation. Heteropoda toxin 2 (150 nM) blocked Ito,epi in a voltage-dependent manner, but had no effect on Ito,endo. Parallel FISH and IF measurements conducted on isolated LV epi and LV endo myocytes demonstrated that Kv1.4, Kv4.2, and Kv4.3 alpha subunit expression in LV myocyte types was quite heterogenous: (a) Kv4.2 and Kv4.3 were more predominantly expressed in LV epi than LV endo myocytes, and (b) Kv1.4 was expressed in the majority of LV endo myocytes but was essentially absent in LV epi myocytes. In combination with previous measurements on recovery kinetics (Kv1.4, slow; Kv4.2/4.3, relatively rapid) and Heteropoda toxin block (Kv1.4, insensitive; Kv4.2, sensitive), our results strongly support the hypothesis that, in ferret heart, Kv4.2/Kv4.3 and Kv1.4 alpha subunits, respectively, are the molecular substrates underlying the Ito,epi and Ito,endo phenotypes. FISH and IF measurements were also conducted on ferret ventricular tissue sections. The three Ito alpha subunits again showed distinct patterns of distribution: (a) Kv1.4 was localized primarily to the apical portion of the LV septum, LV endocardium, and approximate inner 75% of the LV free wall; (b) Kv4. 2 was localized primarily to the right ventricular free wall, epicardial layers of the LV, and base of the heart; and (c) Kv4.3 was localized primarily to epicardial layers of the LV apex and diffusely distributed in the LV free wall and septum. Therefore, in intact ventricular tissue, a heterogeneous distribution of candidate Ito alpha subunits not only exists from LV epicardium to endocardium but also from apex to base.
Figures







References
-
- Antzelevitch, C., S. Sicouri, A. Lukas, V.V. Nesterenko, D.-W. Liu, and J. Di Diego. 1995. Regional differences in the electrophysiology of ventricular cells: physiological and clinical implications. In Cardiac Electrophysiology. From Cell to Bedside. 2nd ed. D. Zipes and J. Jalife, editors. W.B. Saunders Co., Philadelphia. 228–245.
-
- Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annu Rev Physiol. 1996;58:363–394. - PubMed
-
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart: relationship to functional K+ channels? . Circ Res. 1995;77:361–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

